当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revisiting the Determination of Percent Aspirin Lab: Using a Limiting Reactant Approach for Students To Also Determine the Amount of Iron(III) Chloride
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2019-12-30 , DOI: 10.1021/acs.jchemed.9b00154 Matthew Bodek 1 , Mary Burch 1 , Joshua Cannon 1 , David Finneran 2 , Kathleen Geveke 1 , Heather Sinkinson 1 , William Smith 3 , John Tierney 1
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2019-12-30 , DOI: 10.1021/acs.jchemed.9b00154 Matthew Bodek 1 , Mary Burch 1 , Joshua Cannon 1 , David Finneran 2 , Kathleen Geveke 1 , Heather Sinkinson 1 , William Smith 3 , John Tierney 1
Affiliation
|
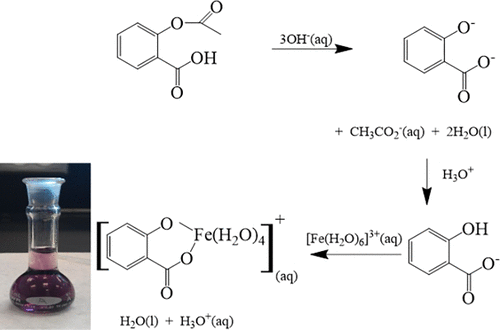
The spectrophotometric determination of the percent composition of an aspirin tablet is a common first-year, general chemistry undergraduate laboratory procedure. The experiment requires that a known amount of pure acetylsalicylic acid be quantitatively converted into the soluble disodium salicylic acid that is further reacted with excess iron(III) chloride solution to form a deep blue tetraaqua-octahedral complex. This complex, through serial dilutions, is used to construct a Beer’s law plot that may be used to determine the concentration of aspirin. In a novel twist, this paper describes how the same Beer’s law plot can be used to determine the concentration of iron in the iron(III) complex, which is then used to determine the concentration of the original iron(III) chloride solution used to make the aspirin–iron(III) complex. The method has been adapted by high school students in determining iron concentrations in stream waters.
中文翻译:

复查阿司匹林实验室的测定方法:使用一种限制性反应物方法,让学生确定氯化铁(III)的量
分光光度法测定阿司匹林片剂的百分组成是一年级通用化学本科实验室的常规操作。实验要求将已知量的纯乙酰水杨酸定量转化为可溶的水杨酸二钠,然后使其与过量的氯化亚铁(III)进一步反应,形成深蓝色的四水八面体络合物。通过连续稀释,该复合物可用于构建比尔定律图,可用于确定阿司匹林的浓度。在新颖的转折中,本文描述了如何使用相同的比尔定律图来确定铁(III)配合物中的铁浓度,然后将其用于确定用于氯化氢的原始氯化铁(III)溶液的浓度。使阿司匹林-铁(III)复合物。
更新日期:2019-12-30
中文翻译:

复查阿司匹林实验室的测定方法:使用一种限制性反应物方法,让学生确定氯化铁(III)的量
分光光度法测定阿司匹林片剂的百分组成是一年级通用化学本科实验室的常规操作。实验要求将已知量的纯乙酰水杨酸定量转化为可溶的水杨酸二钠,然后使其与过量的氯化亚铁(III)进一步反应,形成深蓝色的四水八面体络合物。通过连续稀释,该复合物可用于构建比尔定律图,可用于确定阿司匹林的浓度。在新颖的转折中,本文描述了如何使用相同的比尔定律图来确定铁(III)配合物中的铁浓度,然后将其用于确定用于氯化氢的原始氯化铁(III)溶液的浓度。使阿司匹林-铁(III)复合物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号