当前位置:
X-MOL 学术
›
Drug Metab. Dispos.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Pharmacokinetics, Metabolism, and Clearance Mechanisms of Abrocitinib, a Selective Janus Kinase Inhibitor, in Humans
Drug Metabolism and Disposition ( IF 4.4 ) Pub Date : 2022-08-01 , DOI: 10.1124/dmd.122.000829 Jonathan N Bauman 1 , Angela C Doran 1 , Amanda King-Ahmad 1 , Raman Sharma 1 , Gregory S Walker 1 , Jian Lin 1 , Tsung H Lin 1 , Jean-Baptiste Telliez 1 , Sakambari Tripathy 1 , Theunis C Goosen 1 , Christopher Banfield 1 , Bimal K Malhotra 1 , Martin E Dowty 2
Drug Metabolism and Disposition ( IF 4.4 ) Pub Date : 2022-08-01 , DOI: 10.1124/dmd.122.000829 Jonathan N Bauman 1 , Angela C Doran 1 , Amanda King-Ahmad 1 , Raman Sharma 1 , Gregory S Walker 1 , Jian Lin 1 , Tsung H Lin 1 , Jean-Baptiste Telliez 1 , Sakambari Tripathy 1 , Theunis C Goosen 1 , Christopher Banfield 1 , Bimal K Malhotra 1 , Martin E Dowty 2
Affiliation
|
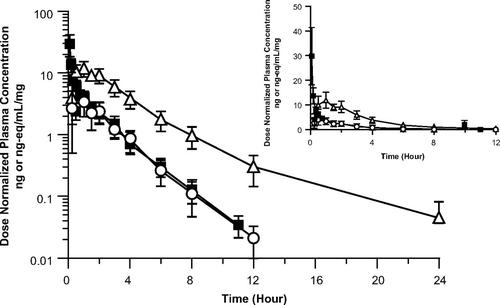
Abrocitinib is an oral once-daily Janus kinase 1 selective inhibitor being developed for the treatment of moderate-to-severe atopic dermatitis. This study examined the disposition of abrocitinib in male participants following oral and intravenous administration using accelerator mass spectroscopy methodology to estimate pharmacokinetic parameters and characterize metabolite (M) profiles. The results indicated abrocitinib had a systemic clearance of 64.2 L/h, a steady-state volume of distribution of 100 L, extent of absorption >90%, time to maximum plasma concentration of ∼0.5 hours, and absolute oral bioavailability of 60%. The half-life of both abrocitinib and total radioactivity was similar, with no indication of metabolite accumulation. Abrocitinib was the main circulating drug species in plasma (∼26%), with 3 major monohydroxylated metabolites (M1, M2, and M4) at >10%. Oxidative metabolism was the primary route of elimination for abrocitinib, with the greatest disposition of radioactivity shown in the urine (∼85%). In vitro phenotyping indicated abrocitinib cytochrome P450 fraction of metabolism assignments of 0.53 for CYP2C19, 0.30 for CYP2C9, 0.11 for CYP3A4, and ∼0.06 for CYP2B6. The principal systemic metabolites M1, M2, and M4 were primarily cleared renally. Abrocitinib, M1, and M2 showed pharmacology with similar Janus kinase 1 selectivity, whereas M4 was inactive.
中文翻译:

选择性 Janus 激酶抑制剂 Abrocitinib 在人体中的药代动力学、代谢和清除机制
Abrocitinib 是一种口服每日一次的 Janus 激酶 1 选择性抑制剂,正在开发用于治疗中度至重度特应性皮炎。本研究使用加速器质谱方法检查了男性参与者口服和静脉内给药后 abrocitinib 的处置情况,以估计药代动力学参数并表征代谢物 (M) 谱。结果表明,阿布罗替尼的全身清除率为 64.2 L/h,稳态分布容积为 100 L,吸收程度为 >90%,达到最大血浆浓度的时间约为 0.5 小时,绝对口服生物利用度为 60%。abrocitinib 和总放射性的半衰期相似,没有代谢物积累的迹象。阿布罗替尼是血浆中主要循环的药物种类 (∼26%),有 3 种主要的单羟基化代谢物 (M1、M2 和 M4) 为 >10%。氧化代谢是 abrocitinib 的主要消除途径,尿液中放射性的分布最大 (∼85%)。体外表型显示阿布罗替尼细胞色素 P450 代谢分配分数为 CYP2C19 为 0.53,CYP2C9 为 0.30,CYP3A4 为 0.11,CYP2B6 为 ∼0.06。主要的全身代谢物 M1 、 M2 和 M4 主要经肾脏清除。Abrocitinib 、 M1 和 M2 的药理学显示具有相似的 Janus 激酶 1 选择性,而 M4 无活性。
更新日期:2022-08-02
中文翻译:

选择性 Janus 激酶抑制剂 Abrocitinib 在人体中的药代动力学、代谢和清除机制
Abrocitinib 是一种口服每日一次的 Janus 激酶 1 选择性抑制剂,正在开发用于治疗中度至重度特应性皮炎。本研究使用加速器质谱方法检查了男性参与者口服和静脉内给药后 abrocitinib 的处置情况,以估计药代动力学参数并表征代谢物 (M) 谱。结果表明,阿布罗替尼的全身清除率为 64.2 L/h,稳态分布容积为 100 L,吸收程度为 >90%,达到最大血浆浓度的时间约为 0.5 小时,绝对口服生物利用度为 60%。abrocitinib 和总放射性的半衰期相似,没有代谢物积累的迹象。阿布罗替尼是血浆中主要循环的药物种类 (∼26%),有 3 种主要的单羟基化代谢物 (M1、M2 和 M4) 为 >10%。氧化代谢是 abrocitinib 的主要消除途径,尿液中放射性的分布最大 (∼85%)。体外表型显示阿布罗替尼细胞色素 P450 代谢分配分数为 CYP2C19 为 0.53,CYP2C9 为 0.30,CYP3A4 为 0.11,CYP2B6 为 ∼0.06。主要的全身代谢物 M1 、 M2 和 M4 主要经肾脏清除。Abrocitinib 、 M1 和 M2 的药理学显示具有相似的 Janus 激酶 1 选择性,而 M4 无活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号