当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The N-terminus of MTRR plays a role in MTR reactivation cycle beyond electron transfer.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-04-10 , DOI: 10.1016/j.bioorg.2020.103836 Jun Zhang 1 , Gui-Cen Liu 2 , Xiao-Lu Dai 2 , Juan Wang 2 , Mu-Hua Jin 2 , Nan-Nan Mi 2 , Shu-Qin Wang 2
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-04-10 , DOI: 10.1016/j.bioorg.2020.103836 Jun Zhang 1 , Gui-Cen Liu 2 , Xiao-Lu Dai 2 , Juan Wang 2 , Mu-Hua Jin 2 , Nan-Nan Mi 2 , Shu-Qin Wang 2
Affiliation
|
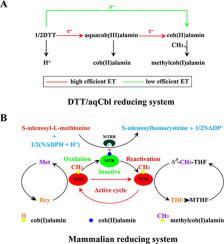
In eucaryotic cells, methionine synthase reductase (MSR/MTRR) is capable of dominating the folate-homocysteine metabolism as an irreplaceable partner in electron transfer for regeneration of methionine synthase. The N-terminus of MTRR containing a conserved domain of FMN_Red is closely concerned with the oxidation-reduction process. Maternal substitution of I22M in this domain can bring about pregnancy with high risk of spina bifida. A new variation of Arg2del was identified from a female conceiving a fetus with spina bifida cystica. Although the deletion is far from the N-terminal FMN_Red domain, the biochemical features of the variant had been seriously investigated. Curiously, the deletion of arginine(s) of MTRR could not affect the electron relay, if only the FMN_Red domain was intact, but by degrees reduced the ability to promote MTR catalysis in methionine formation. Confirmation of the interaction between the isolated MTRR N-terminal polypeptide and MTR suggested that the native MTRR N-terminus might play an extra role in MTR function. The tandem arginines at the end of MTRR N-terminus conferring high affinity to MTR were indispensable for stimulating methyltransferase activity perhaps via triggering allosteric effect that could be attenuated by removal of the arginine(s). It was concluded that MTRR could also propel MTR enzymatic reaction relying on the tandem arginines at N-terminus more than just only implicated in electron transfer in MTR reactivation cycle. Perturbance of the enzymatic cooperation due to the novel deletion could possibly invite spina bifida in clinics.
中文翻译:

MTRR 的 N 末端在电子转移之外的 MTR 再激活循环中发挥作用。
在真核细胞中,蛋氨酸合酶还原酶 (MSR/MTRR) 能够主导叶酸-同型半胱氨酸代谢,作为蛋氨酸合酶再生的电子转移中不可替代的伙伴。包含 FMN_Red 保守结构域的 MTRR N 末端与氧化还原过程密切相关。母体在该域中替代 I22M 可导致怀孕,脊柱裂的风险很高。Arg2del 的一个新变种是从一名怀有囊性脊柱裂胎儿的女性身上发现的。尽管缺失远离 N 端 FMN_Red 结构域,但已认真研究了该变体的生化特征。奇怪的是,MTRR 精氨酸的删除不会影响电子中继,只要 FMN_Red 域完好无损,但逐渐降低了促进 MTR 催化甲硫氨酸形成的能力。确认分离的 MTRR N-末端多肽和 MTR 之间的相互作用表明天然 MTRR N-末端可能在 MTR 功能中发挥额外作用。MTRR N 末端末端的串联精氨酸赋予 MTR 高亲和力,这对于刺激甲基转移酶活性是必不可少的,这可能是通过触发可通过去除精氨酸而减弱的变构效应。得出的结论是,MTRR 还可以推动依赖于 N 末端串联精氨酸的 MTR 酶促反应,而不仅仅是与 MTR 再激活循环中的电子转移有关。由于新的缺失引起的酶促合作的干扰可能会在临床上引起脊柱裂。确认分离的 MTRR N-末端多肽和 MTR 之间的相互作用表明天然 MTRR N-末端可能在 MTR 功能中发挥额外作用。MTRR N 末端末端的串联精氨酸赋予 MTR 高亲和力,这对于刺激甲基转移酶活性是必不可少的,这可能是通过触发可通过去除精氨酸而减弱的变构效应。得出的结论是,MTRR 还可以推动依赖于 N 末端串联精氨酸的 MTR 酶促反应,而不仅仅是与 MTR 再激活循环中的电子转移有关。由于新的缺失引起的酶促合作的干扰可能会在临床上引起脊柱裂。确认分离的 MTRR N-末端多肽和 MTR 之间的相互作用表明天然 MTRR N-末端可能在 MTR 功能中发挥额外作用。MTRR N 末端末端的串联精氨酸赋予 MTR 高亲和力,这对于刺激甲基转移酶活性是必不可少的,这可能是通过触发可通过去除精氨酸而减弱的变构效应。得出的结论是,MTRR 还可以推动依赖于 N 末端串联精氨酸的 MTR 酶促反应,而不仅仅是与 MTR 再激活循环中的电子转移有关。由于新的缺失引起的酶促合作的干扰可能会在临床上引起脊柱裂。
更新日期:2020-04-10
中文翻译:

MTRR 的 N 末端在电子转移之外的 MTR 再激活循环中发挥作用。
在真核细胞中,蛋氨酸合酶还原酶 (MSR/MTRR) 能够主导叶酸-同型半胱氨酸代谢,作为蛋氨酸合酶再生的电子转移中不可替代的伙伴。包含 FMN_Red 保守结构域的 MTRR N 末端与氧化还原过程密切相关。母体在该域中替代 I22M 可导致怀孕,脊柱裂的风险很高。Arg2del 的一个新变种是从一名怀有囊性脊柱裂胎儿的女性身上发现的。尽管缺失远离 N 端 FMN_Red 结构域,但已认真研究了该变体的生化特征。奇怪的是,MTRR 精氨酸的删除不会影响电子中继,只要 FMN_Red 域完好无损,但逐渐降低了促进 MTR 催化甲硫氨酸形成的能力。确认分离的 MTRR N-末端多肽和 MTR 之间的相互作用表明天然 MTRR N-末端可能在 MTR 功能中发挥额外作用。MTRR N 末端末端的串联精氨酸赋予 MTR 高亲和力,这对于刺激甲基转移酶活性是必不可少的,这可能是通过触发可通过去除精氨酸而减弱的变构效应。得出的结论是,MTRR 还可以推动依赖于 N 末端串联精氨酸的 MTR 酶促反应,而不仅仅是与 MTR 再激活循环中的电子转移有关。由于新的缺失引起的酶促合作的干扰可能会在临床上引起脊柱裂。确认分离的 MTRR N-末端多肽和 MTR 之间的相互作用表明天然 MTRR N-末端可能在 MTR 功能中发挥额外作用。MTRR N 末端末端的串联精氨酸赋予 MTR 高亲和力,这对于刺激甲基转移酶活性是必不可少的,这可能是通过触发可通过去除精氨酸而减弱的变构效应。得出的结论是,MTRR 还可以推动依赖于 N 末端串联精氨酸的 MTR 酶促反应,而不仅仅是与 MTR 再激活循环中的电子转移有关。由于新的缺失引起的酶促合作的干扰可能会在临床上引起脊柱裂。确认分离的 MTRR N-末端多肽和 MTR 之间的相互作用表明天然 MTRR N-末端可能在 MTR 功能中发挥额外作用。MTRR N 末端末端的串联精氨酸赋予 MTR 高亲和力,这对于刺激甲基转移酶活性是必不可少的,这可能是通过触发可通过去除精氨酸而减弱的变构效应。得出的结论是,MTRR 还可以推动依赖于 N 末端串联精氨酸的 MTR 酶促反应,而不仅仅是与 MTR 再激活循环中的电子转移有关。由于新的缺失引起的酶促合作的干扰可能会在临床上引起脊柱裂。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号