EB病毒(EBV,Epstein-Barr virus)能导致一系列疾病发生,特别是慢性EBV感染易引发癌症如鼻咽癌、淋巴癌等。近日,一篇发表在国际杂志Science上题为“A metabolic dependency of EBV can be targeted to hinder B cell transformation”的研究论文中,来自巴塞尔大学等机构的科学家们通过研究发现,抑制感染细胞中的特殊代谢途径或能减少EB病毒的潜伏感染,从而降低个体患下游疾病的风险。

整整60年前,病理学家Anthony Epstein和病毒学家Yvonne Barr宣布他们发现了一种新的病毒,并以他们的名字命名,EBV——作为第一种被证实能够在人类引起癌症的病毒,创造了科学史,上述两位研究人员成功从肿瘤组织样本中分离到了EBV,其是疱疹病毒家族的一份子,同时还在后续的实验中阐明了EBV潜在的致癌潜力。
听着很可怕?其实,我们大多数人群都是EBV的携带者,90%的成年人都感染过这种病毒,不过,大部分的感染者并没有明显疾病症状,也不会引起疾病发生。并且,大约50%的人群会在5岁之前就会经历EB病毒感染。该病毒引起的急性感染会引起传染性单核细胞增多症(glandular fever),也就是我们俗称的接吻病,其会让感染者丧失数月的活动能力,除了其具有一定的致癌特性外,EBV还被推测参与到了诸如多发性硬化症等人类自身免疫性疾病的发病过程中。
然而到目前为止,并没有药物或获批的疫苗能特异性地阻断机体中的EBV感染。这项研究中,研究人员报告了一种能遏制EBV的非常有希望的起点。研究人员解析了被EBV感染的B细胞重编程的分子机制,这一过程称之为“转化”(transformation),即感染变成慢性疾病并导致诸如癌症等后续疾病的必要条件。
具体而言,研究人员发现,病毒能诱发感染的细胞会加速产生名为吲哚胺2,3-双加氧酶1(indoleamine 2,3-dioxygenase 1 ,IDO1)的酶类,导致线粒体产生更多的能量,而这些额外的能量正是EBV重编程B细胞增加其代谢和快速增殖所需要的。
在临床上,研究人员重点研究了器官移植后患上EBV所诱导的血液癌症患者,为了防止移植的器官被机体排斥,研究人员就需要利用药物来削弱宿主机体免疫系统的功能,这就会让EBV更容易占据上风并引起血液癌症,即移植后淋巴瘤(post-transplant lymphoma)。
文章中,研究人员发现,早在个体被诊断为移植后淋巴瘤的数月前,潜伏在机体中的EBV就已经开始上调酶类IDO1的表达了,这一研究发现或能帮助开发针对这一疾病的生物标志物。研究者Hess解释道,此前,我们希望IDO1抑制剂能帮助治疗癌症,但不幸的是,事实并非如此,换句话说,目前已经有针对这种酶类的临床试验抑制剂了。
因此,这类药物有望第二次应用来减弱EBV的感染,从而治疗EBV相关疾病。目前,在小鼠实验中,利用这些药物能抑制IDO1或能减少B细胞的转化,从而减少病毒载量和淋巴瘤的发展。
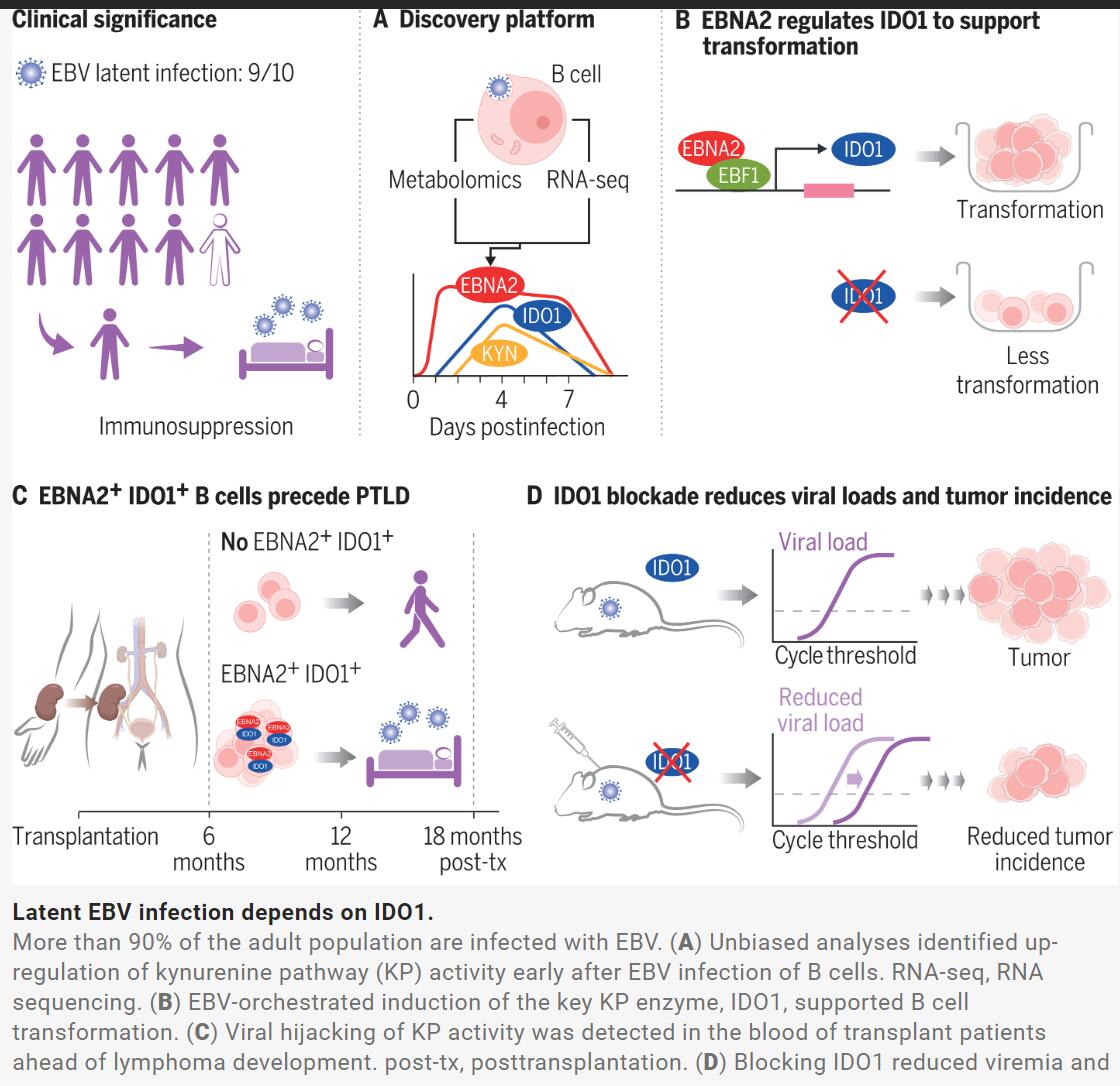
总结而言,本研究表明,在模拟人类EBV感染环境的小鼠模型中,通过抑制IDO1酶的活性,不仅能有效控制病毒在血液中的活跃程度(病毒血症),还能显著抑制淋巴瘤的形成。
这项发现揭示了EB病毒利用NAD生物合成途径促进B细胞恶性转化的代谢弱点,为EBV相关疾病的治疗提供了创新思路。通过针对性干预这一代谢通路,我们向前迈出了关键一步,开辟了利用IDO1抑制剂等代谢靶向疗法来治疗EB病毒引发的多种疾病的可能性,为患者带来了新的治疗希望。
编辑总结Editor’s summary
Epstein-Barr virus (EBV) is a prevalent, persistent infection in humans. Although many cases are asymptomatic, EBV may cause disease in individuals with a weakened immune system. Müller-Durovic et al. found that the virally encoded protein EBNA2 induced the expression of the metabolic enzyme indoleamine 2,3-dioxygenase 1 (IDO1) in infected B cells and sustained their subsequent proliferation and transformation into malignant cells. In transplant recipients, elevated expression of IDO1 in B cells preceded the development of lymphoma. Treating mice with a small-molecule inhibitor of IDO1 before infection with EBV decreased the development of tumors, suggesting that blocking the pathway might be a strategy for protecting EBV-negative individuals receiving solid organ transplants. —Sarah H. Ross
Epstein-Barr病毒(EBV)是人类普遍存在的持续性感染。尽管许多病例无症状,但在免疫系统受损的个体中,EBV可能引起疾病。Müller-Durovic等人发现,病毒编码的蛋白EBNA2在感染的B细胞中诱导了代谢酶吲哚胺2,3-双加氧酶1(IDO1)的表达,并维持了它们随后的增殖和转化为恶性细胞。在移植受者中,B细胞中IDO1的表达升高先于淋巴瘤的发展。在感染EBV之前,用小分子IDO1抑制剂治疗小鼠减少了肿瘤的发展,表明阻断这一途径可能是保护接受实体器官移植的EBV阴性个体的策略。—Sarah H. Ross
结构性摘要Abstract
INTRODUCTION 引言
More than 90% of the adult population worldwide are infected with the Epstein-Barr virus (EBV). EBV, which belongs to the γ-herpes virus family, is transferred through saliva, and, if symptomatic, de novo infection causes infectious mononucleosis. The virus is B cell tropic and establishes lifelong latent infection in this cellular compartment. Originally, EBV was identified in Burkitt’s lymphoma cells, and the virus is well known for its growth-transforming and tumorigenic properties. EBV can also cause life-threatening hyperinflammation (hemophagocytic lymphohistiocytosis) and has also been strongly associated with the development of autoimmunity (e.g., multiple sclerosis and systemic lupus erythematosus). When infecting B cells, EBV causes their rapid growth and proliferation, a metabolically demanding process that is a prerequisite for establishing latency.
全球超过90%的成年人口感染了Epstein-Barr病毒(EBV)。EBV属于γ-疱疹病毒科,通过唾液传播,如果出现症状,新的感染会导致传染性单核细胞增多症。该病毒对B细胞具有特异性,并在这一细胞群中建立了终生潜伏感染。最初,EBV是在Burkitt淋巴瘤细胞中被鉴定出来的,该病毒以其生长转化和肿瘤特性而闻名。EBV还可能引起危及生命的超炎症反应(血细胞吞噬性淋巴组织细胞增多症),并且与自身免疫疾病的发展(例如,多发性硬化症和系统性红斑狼疮)有很强的关联。当EBV感染B细胞时,会导致它们迅速生长和增殖,这是一个对代谢要求很高的过程,是建立潜伏感染的先决条件。
RATIONALE
The metabolic challenge that EBV imposes on nascently infected B cells may create bottlenecks for the virus en route to establishing latent infection. Targeting such early host cell metabolic dependencies of EBV could hinder progression to latency, the infection stage underpinning most EBV-related pathologies.
EBV对新感染的B细胞造成的代谢挑战可能会在建立潜伏感染的过程中为病毒带来瓶颈。针对EBV早期宿主细胞代谢依赖性可能阻碍其向潜伏期的进展,这是大多数EBV相关病理的基础感染阶段。
RESULTS结果
We performed unbiased metabolomic and transcriptome-based analyses and found evidence that the activity of the kynurenine pathway and the nicotinamide adenine dinucleotide (NAD) de novo synthesis pathway were up-regulated in B cells during the prelatency phase after EBV infection. We discovered that the viral protein, EBV-encoded transactivator EBNA2, in cooperation with the host B cell transcription factor EBF1, drove induction of indoleamine 2,3-dioxygenase 1 (IDO1), the first and rate-limiting enzyme of the kynurenine pathway. IDO1-dependent degradation of tryptophan fueled NAD de novo synthesis, which supported mitochondrial adenosine triphosphate production in the early phase of EBV infection. Pharmacologic inhibition of IDO1 rendered B cells up to 100-fold less susceptible to EBV transformation, a hurdle that was removed by adding back the product of IDO1, kynurenine, as well as by supplementing nicotinic acid mononucleotide, the direct precursor of NAD.
In the peripheral blood of patients that eventually developed posttransplant lymphoma, we identified a population of B cells that expressed EBNA2 as well as IDO1, suggesting that, before development of EBV-driven malignancy, the molecular axis we uncovered in vitro may be engaged in vivo. In mice, blocking IDO1 pharmacologically, or deleting IDO1 genetically in B cells, reduced EBV viral loads and inhibited the formation of B cell tumors.
作者进行了无偏见的代谢组学和基于转录组的分析,并发现在EBV感染后的前潜伏期,B细胞中犬尿氨酸途径和烟酰胺腺嘌呤二核苷酸(NAD)从头合成途径被激活。作者发现,EBV编码的转录激活因子EBNA2与宿主B细胞转录因子EBF1协同作用,诱导了犬尿氨酸途径的第一个限速酶吲哚胺2,3-双加氧酶1(IDO1)的表达。IDO1依赖的色氨酸降解促进了NAD新合成,这支持了EBV感染早期阶段线粒体腺苷三磷酸的产生。药理学抑制IDO1使B细胞对EBV转化的敏感性降低了100倍,通过添加IDO1的产物犬尿氨酸以及补充烟酸单核苷酸,NAD的直接前体,可以消除这一障碍。
在最终发展为移植后淋巴瘤的患者的外周血中,作者鉴定了一群表达EBNA2和IDO1的B细胞,这表明在EBV驱动的恶性病变发展之前,我们在体外发现的分子轴可能在体内被激活。在小鼠中,药理学阻断IDO1或敲除B细胞中IDO1基因,减少了EBV病毒载量并抑制了B细胞肿瘤的形成。
CONCLUSION 结论
Early after infecting B cells, EBV induced the expression of IDO1 in host cells, which changed the activity of B cell metabolic pathways. Hijacking this host axis was a metabolic requirement for the virus to efficiently establish latent infection, in vitro and in vivo. Targeting IDO1 clinically may thus offer an opportunity to interfere with progression of EBV infection in B cells from prelatency to latency. EBV-naïve solid organ transplant recipients are at a very high risk for developing posttransplantation lymphoproliferative disorders (PTLDs), particularly when EBV-negative recipients receive tissue from EBV-positive donors. In this context, pharmacological inhibition of IDO1 could limit EBV-driven pathology. Precisely defining metabolic bottlenecks that viruses have evolved to depend upon in vivo may identify druggable targets beyond IDO1 in other settings.
EBV在感染B细胞后早期诱导宿主细胞中代谢酶IDO1的表达,这改变了B细胞代谢途径的活性。劫持宿主信号轴是病毒有效建立潜伏感染的代谢需求,无论是体外还是体内。因此,临床上针对IDO1可能提供了一个干扰EBV感染从前潜伏期到潜伏期进展的机会。EBV阴性的实体器官移植受者在发展移植后增生性疾病(PTLD)方面风险极高,特别是当EBV阴性受者接受EBV阳性供者的组织时。在这种情况下,药理学抑制IDO1可能限制EBV驱动的病理学。此外,精确定义病毒在体内进化依赖的代谢瓶颈 可能有助于识别IDO1之外的其他情况下的药物靶标。
DOI: 10.1126/science.adk4898
原文链接:https://www.science.org/doi/abs/10.1126/science.adk4898
参考文献:BOJANA MÜLLER-DUROVIC,JESSICA JÄGER,CHRISTINE ENGELMANN, et al. A metabolic dependency of EBV can be targeted to hinder B cell transformation, Science (2024).