2024年3月1日,内布拉斯加大学医学中心Pankaj K. Singh研究团队在 Nature cell biology 期刊在线发表题为“Cancer-associated fibroblast-derived acetate promotes pancreatic cancer development by altering polyamine metabolism via the ACSS2–SP1–SAT1 axis”的论文,改研究发现胰腺癌相关的成纤维细胞CAF分泌乙酸,重塑肿瘤表观遗传景观,以多胺代谢等方式促进肿瘤生长。同时, Nature cell biology 网站的NEWS&VIEW栏目对此进行了点评“Targeting stromal metabolism in pancreatic ductal adenocarcinoma”。
研究人员观察到CAF分泌乙酸,而乙酸是支持胰腺癌症细胞生长的关键代谢产物。在酸中毒情况下,乙酸通过ACSS2改变组蛋白乙酰化而诱导肿瘤细胞的表观遗传重编程,这对胰腺癌细胞的存活至关重要。而 乙酰辅酶A合成酶2(ACSS2)在肿瘤的酸性区域高度表达,提示了肿瘤对酸性环境的局部代谢反应。 CAF中ATP柠檬酸裂解酶( ATP citrate lyase, ACLY)的缺失显著减少了乙酸的分泌,从而抑制胰腺癌症细胞的生长。 H3K27ac ChIP–seq 与RNA–seq 联合分析发现,多胺代谢酶——亚精胺/精胺N1-乙酰转移酶1(Spermidine/Spermine N1-Acetyltransferase 1,SAT1)的表达改变与多胺代谢重塑。进一步机制研究发现,转录因子SP1的赖氨酸19残基位点上鉴定了一个乙酰化位点,这对乙酸介导的SP1的稳定性至关重要。该乙酰化修饰增强了SP1的蛋白质稳定性和转录活性,从而诱导SAT1的表达上调。 最后,通过抑制SP1,能够减少CAF诱导的小鼠体内肿瘤负荷,表明靶向“ACSS2–SP1–SAT1”轴可以为癌症治疗提供新的策略。
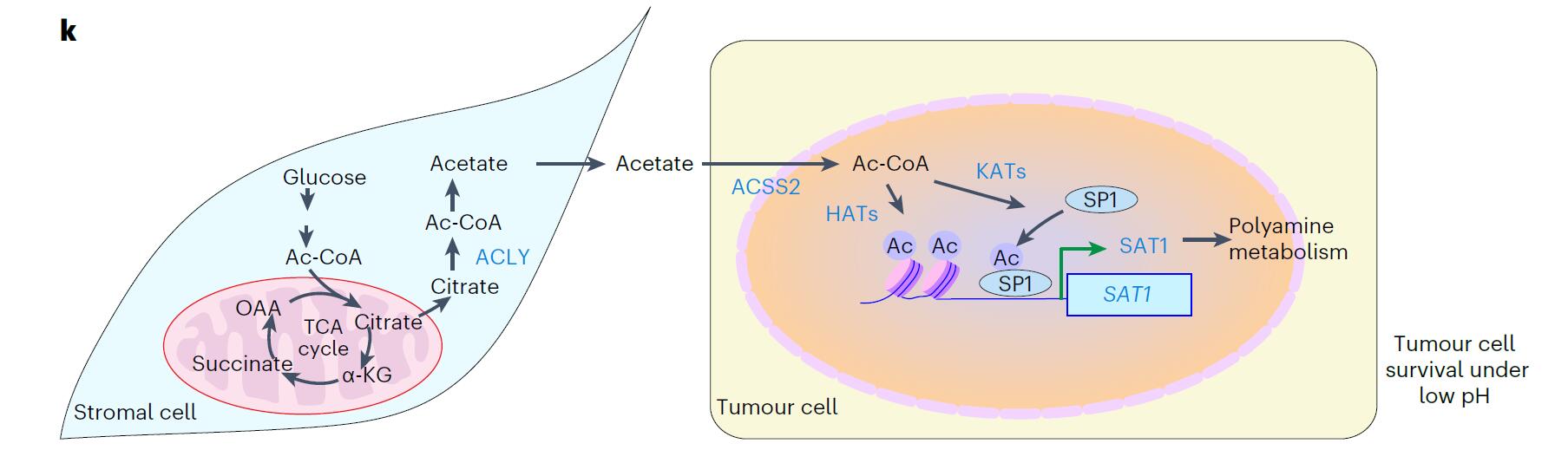
总之,该研究为胰腺癌细胞及其基质环境之间的代谢互动提供了新的见解,描绘了胰腺癌中的代谢重编程与表观遗传重编程之间的交互调控,揭示了CAF通过分泌乙酸影响肿瘤生长的机制。
原文链接:https://www.nature.com/articles/s41556-024-01372-4
原文摘要:Abstract
The ability of tumour cells to thrive in harsh microenvironments depends on the utilization of nutrients available in the milieu. Here we show that pancreatic cancer-associated fibroblasts (CAFs) regulate tumour cell metabolism through the secretion of acetate, which can be blocked by silencing ATP citrate lyase (ACLY) in CAFs. We further show that acetyl-CoA synthetase short-chain family member 2 (ACSS2) channels the exogenous acetate to regulate the dynamic cancer epigenome and transcriptome, thereby facilitating cancer cell survival in an acidic microenvironment. Comparative H3K27ac ChIP–seq and RNA–seq analyses revealed alterations in polyamine homeostasis through regulation of SAT1 gene expression and enrichment of the SP1-responsive signature. We identified acetate/ACSS2-mediated acetylation of SP1 at the lysine 19 residue that increased SP1 protein stability and transcriptional activity. Genetic or pharmacologic inhibition of the ACSS2–SP1–SAT1 axis diminished the tumour burden in mouse models. These results reveal that the metabolic flexibility imparted by the stroma-derived acetate enabled cancer cell survival under acidosis via the ACSS2–SP1–SAT1 axis.
DOI: https://doi.org/10.1038/s41556-024-01372-4
述评文章:Targeting stromal metabolism in pancreatic ductal adenocarcinoma https://www.nature.com/articles/s41556-023-01330-6
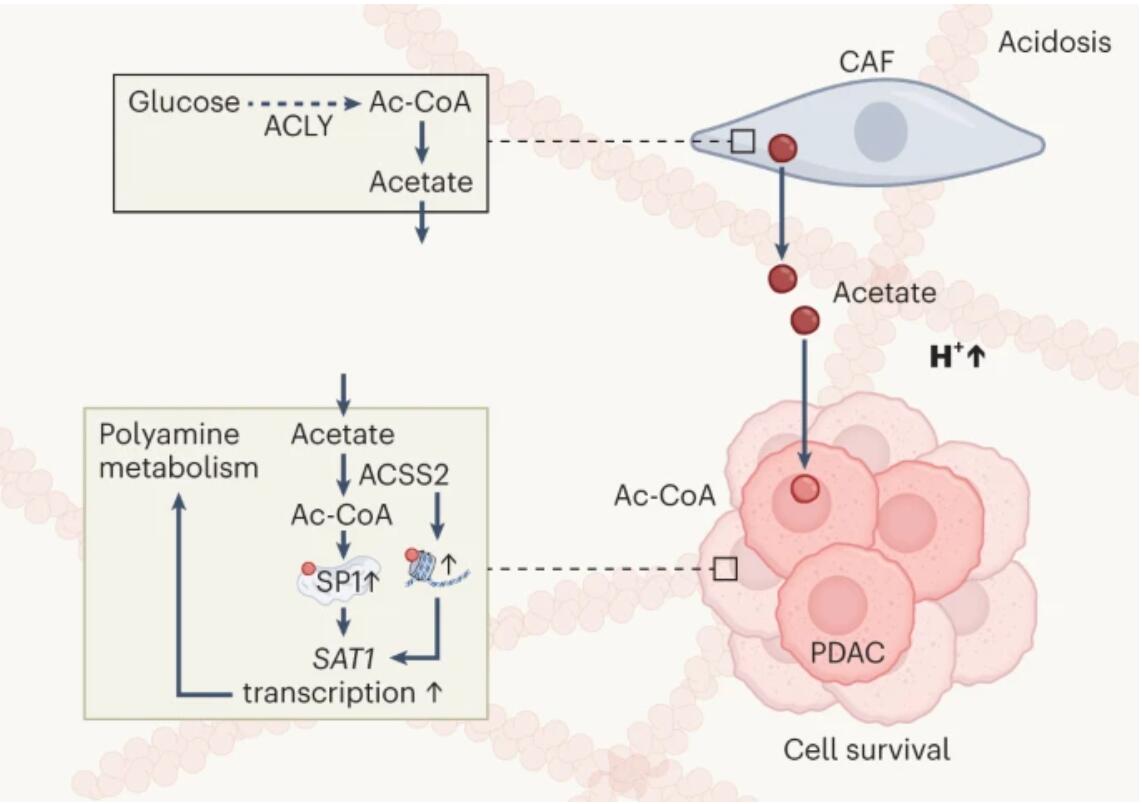
In a tumour microenvironment with low pH, CAFs with functional ACLY protein feed PDAC cells by producing and secreting acetate (larger orange circles). In PDAC cells, acetate is catalysed by ACSS2 to produce acetyl-CoA (Ac-CoA; small red circles), resulting in upregulation of transcription of SAT1 through epigenetic modification and through stabilization of the transcription factor SP1. This in turn drives polyamine metabolism. H+, proton.
参考文献:
Murthy, D., Attri, K.S., Shukla, S.K. et al. Cancer-associated fibroblast-derived acetate promotes pancreatic cancer development by altering polyamine metabolism via the ACSS2–SP1–SAT1 axis. Nat Cell Biol (2024). https://doi.org/10.1038/s41556-024-01372-4
Yin, M., Lei, QY. Targeting stromal metabolism in pancreatic ductal adenocarcinoma. Nat Cell Biol (2024). https://doi.org/10.1038/s41556-023-01330-6