大多数细胞的生长和增殖都依赖于细胞自主合成的天冬氨酸。天冬氨酸的合成和消耗是由两种可逆反应控制的——其合成是在线粒体中由谷氨酸和草酰乙酸通过谷氨酸-草酰乙酸转氨酶2(GOT2)实现的,而其进入细胞质后则被谷氨酸-草酰乙酸转氨酶1(GOT1)所消耗。谷草转氨酶(GOT),又叫天冬氨酸转氨酶(AST),是转氨酶中比较重要的一种。肝内的谷草转氨酶有2种同工酶,分别存在于肝细胞的线粒体(mAST)和胞浆内(sAST)。在肝细胞轻度病变时,仅sAST释放入血;而当病变严重时,mAST也会相继释放入血。故血清谷草转氨酶的数值随肝细胞损害的程度增高。在对药物副作用的监测中,也是一个敏感的指标。
近期三项研究报道了谷氨酸草酰乙酸转氨酶2(GOT2)与PDAC肿瘤微环境,为这种致命癌症的代谢提供了新的见解。谷草转氨酶GOT2参与谷氨酰胺代谢,介导谷氨酸和草酰乙酸转化为α-酮戊二酸和天冬氨酸。
To thrive in a hypoxic and nutrient-limited tumor microenvironment, pancreatic ductal adenocarcinoma (PDAC) cells rewire their metabolism. Understanding PDAC cell metabolism may uncover vulnerabilities that can be targeted for improved therapy. Three recent studies find that the PDAC tumor microenvironment modulates the functional consequences of depleting the mitochondrially localized aspartate transaminase GOT2, thus providing new insights into the metabolism of this lethal cancer 【1】.
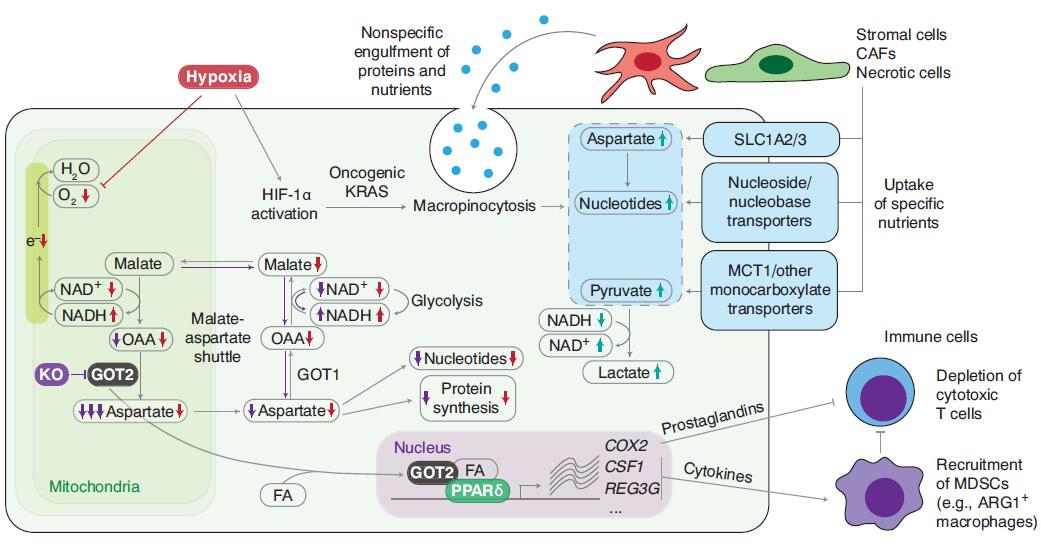
谷氨酸草酰乙酸转氨酶2与肿瘤微环境之间的相互作用
Interplay between pathways involving the aspartate transaminase GOT2 and the tumor microenvironment (DOI: https://doi.org/10.1016/j.trecan.2022.09.004 )
第一篇:Adaptive stimulation of macropinocytosis overcomes aspartate limitation in cancer cells under hypoxia
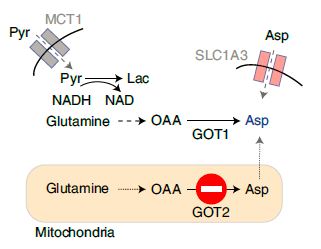
Abstract:Stress-adaptive mechanisms enable tumour cells to overcome metabolic constraints under nutrient and oxygen shortage. Aspartate is an endogenous metabolic limitation under hypoxic conditions, but the nature of the adaptive mechanisms that contribute to aspartate availability and hypoxic tumour growth are poorly understood. Here we identify GOT2-catalysed mitochondrial aspartate synthesis as an essential metabolic dependency for the proliferation of pancreatic tumour cells under hypoxic culture conditions. In contrast, GOT2-catalysed aspartate synthesis is dispensable for pancreatic tumour formation in vivo. The dependence of pancreatic tumour cells on aspartate synthesis is bypassed in part by a hypoxia-induced potentiation of extracellular protein scavenging via macropinocytosis. This effect is mutant KRAS dependent, and is mediated by hypoxia-inducible factor 1 (HIF1A) and its canonical target carbonic anhydrase-9 (CA9). Our findings reveal high plasticity of aspartate metabolism and define an adaptive regulatory role for macropinocytosis by which mutant KRAS tumours can overcome nutrient deprivation under hypoxic conditions.【2】
第二篇:A Cancer Cell–Intrinsic GOT2–PPARδ Axis Suppresses Antitumor Immunity
Abstract:Despite significant recent advances in precision medicine, pancreatic ductal adenocarcinoma (PDAC) remains near uniformly lethal. Although immune-modulatory therapies hold promise to meaningfully improve outcomes for patients with PDAC, the development of such therapies requires an improved understanding of the immune evasion mechanisms that characterize the PDAC microenvironment. Here, we show that cancer cell–intrinsic glutamic-oxaloacetic transaminase 2 (GOT2) shapes the immune microenvironment to suppress antitumor immunity. Mechanistically, we find that GOT2 functions beyond its established role in the malate–aspartate shuttle and promotes the transcriptional activity of nuclear receptor peroxisome proliferator–activated receptor delta (PPARδ), facilitated by direct fatty acid binding. Although GOT2 is dispensable for cancer cell proliferation in vivo, the GOT2–PPARδ axis promotes spatial restriction of both CD4+ and CD8+ T cells from the tumor microenvironment. Our results demonstrate a noncanonical function for an established mitochondrial enzyme in transcriptional regulation of immune evasion, which may be exploitable to promote a productive antitumor immune response.
Significance: Prior studies demonstrate the important moonlighting functions of metabolic enzymes in cancer. We find that the mitochondrial transaminase GOT2 binds directly to fatty acid ligands that regulate the nuclear receptor PPARδ, and this functional interaction critically regulates the immune microenvironment of pancreatic cancer to promote tumor progression.【3】
第三篇:Metabolic requirement for GOT2 in pancreatic cancer depends on environmental context
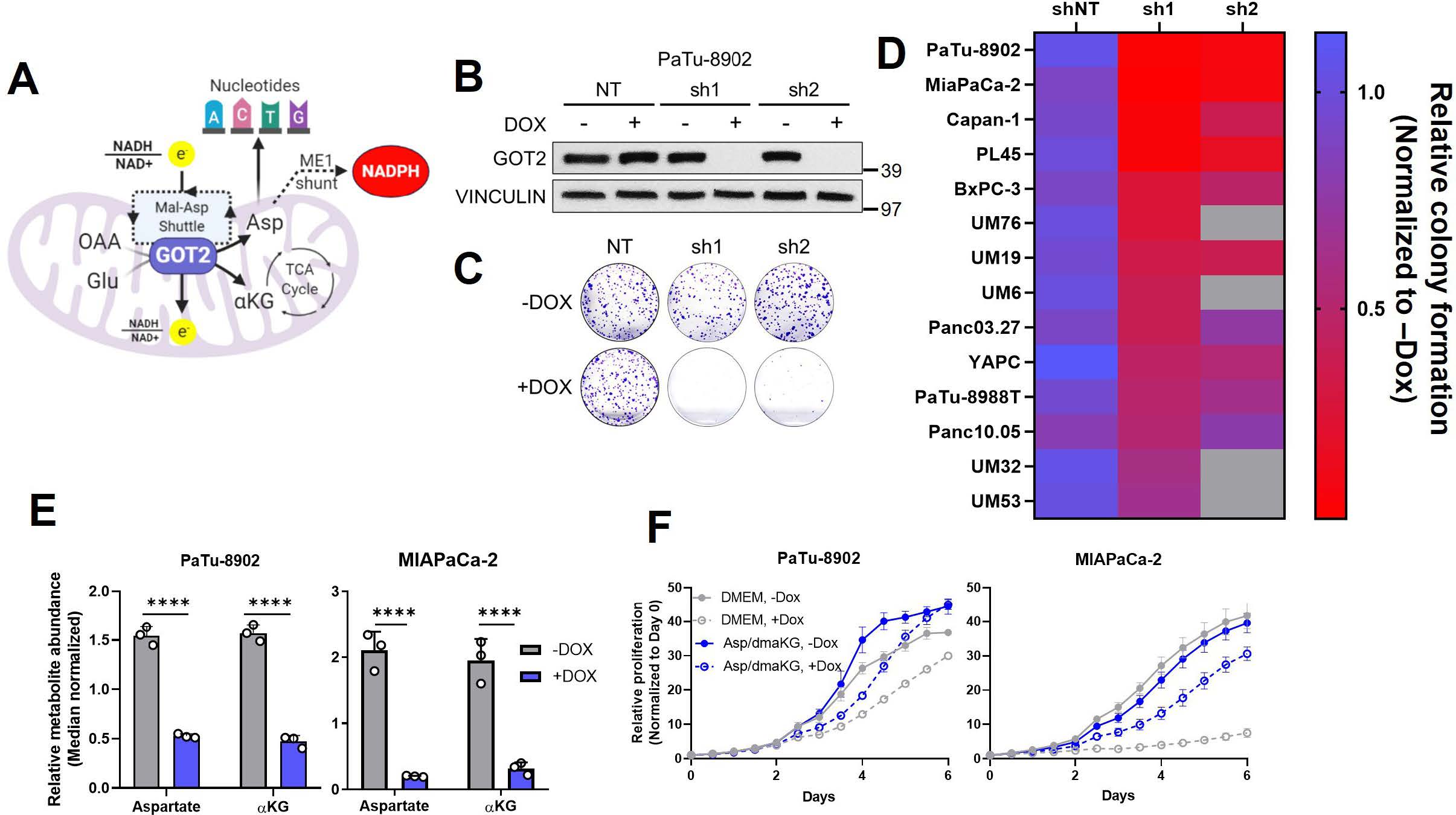
Abstract: Mitochondrial glutamate-oxaloacetate transaminase 2 (GOT2) is part of the malate-aspartate shuttle, a mechanism by which cells transfer reducing equivalents from the cytosol to the mitochondria. GOT2 is a key component of mutant KRAS (KRAS*)-mediated rewiring of glutamine metabolism in pancreatic ductal adenocarcinoma (PDA). Here, we demonstrate that the loss of GOT2 disturbs redox homeostasis and halts proliferation of PDA cells in vitro. GOT2 knockdown (KD) in PDA cell lines in vitro induced NADH accumulation, decreased Asp and α-ketoglutarate (αKG) production, stalled glycolysis, disrupted the TCA cycle, and impaired proliferation. Oxidizing NADH through chemical or genetic means resolved the redox imbalance induced by GOT2 KD, permitting sustained proliferation. Despite a strong in vitro inhibitory phenotype, loss of GOT2 had no effect on tumor growth in xenograft PDA or autochthonous mouse models. We show that cancer-associated fibroblasts (CAFs), a major component of the pancreatic tumor microenvironment (TME), release the redox active metabolite pyruvate, and culturing GOT2 KD cells in CAF conditioned media (CM) rescued proliferation in vitro. Furthermore, blocking pyruvate import or pyruvate-to-lactate reduction prevented rescue of GOT2 KD in vitro by exogenous pyruvate or CAF CM. However, these interventions failed to sensitize xenografts to GOT2 KD in vivo, demonstrating the remarkable plasticity and differential metabolism deployed by PDA cells in vitro and in vivo. This emphasizes how the environmental context of distinct pre-clinical models impacts both cell-intrinsic metabolic rewiring and metabolic crosstalk with the TME.【4】
新陈代谢是所有生物能源和原材料的来源。病毒感染需要改变宿主细胞代谢网络获得病毒生存的物质能量。例如,正链RNA病毒复制需要包膜病毒重建脂质代谢,丙型肝炎病毒在肝细胞触发谷氨酰胺代谢。尽管一些研究发现,宿主或病毒编码的microRNAs 具有调节宿主细胞代谢的作用,但是其分子机制仍然存在许多未知。尤其是病毒如何主动改变宿主细胞状态以便于逃逸免疫清除、进而利于病毒本身复制和存活。这仍然是免疫学与病毒学竞相研究的重要科学问题。
曹雪涛院士与海军军医大学医学免疫学国家重点实验室王品副教授、浙江大学医学院免疫学研究所博士生徐俊芳等研究表明,lncRNA-ACOD1通过直接结合谷氨酸-草酰乙酸转氨酶(GOT2)促进代谢活性,改变细胞代谢状态以促进病毒复制。该研究提出了病毒感染如何以主动性反馈方式、通过表观遗传机制调控宿主免疫细胞的代谢状态而利于病毒自身存活的新观点,揭示了表观遗传、细胞代谢和病毒感染之间的新调控网络,为病毒与宿主相互作用以及病毒免疫逃逸的未来研究提出了新的研究方向【5】。
主要参考文献:
1. Brian T. Do, Matthew G. Vander Heiden. GOT2 consider the tumor microenvironment https://www.cell.com/trends/cancer/fulltext/S2405-8033(22)00195-9
2. Garcia-Bermudez J. et al. Adaptive stimulation of macropinocytosis overcomes aspartate limitation in cancer cells under hypoxia. Nat. Metab. 2022; 4: 724-738 https://www.nature.com/articles/s42255-022-00583-z
3. Abrego J. et al. A cancer cell-intrinsic GOT2–PPARd axis suppresses antitumor immunity.
Cancer Discov. 2022; (Published online July 27, 2022) https://doi.org/10.1158/2159-8290.CD-22-0661
4. Kerk S.A. et al. Metabolic requirement for GOT2 in pancreatic cancer depends on environmental context. eLife. 2022; 11e73245 https://elifesciences.org/articles/73245
5. PinWang, Junfang Xu, Yujia Wang, et al. An interferon-independent lncRNA promotesviral replication by modulating cellular metabolism. Science. 26 Oct 2017:DOI:10.1126/science.aao0409A