EMBO Reports ( IF 6.5 ) Pub Date : 2018-12-01 , DOI: 10.15252/embr.201846363
Norihiko Furuya 1, 2, 3 , Soichiro Kakuta 4, 5 , Katsuhiko Sumiyoshi 6, 7 , Maya Ando 3 , Risa Nonaka 8 , Ayami Suzuki 3 , Saiko Kazuno 9 , Shinji Saiki 1, 3 , Nobutaka Hattori 3
|
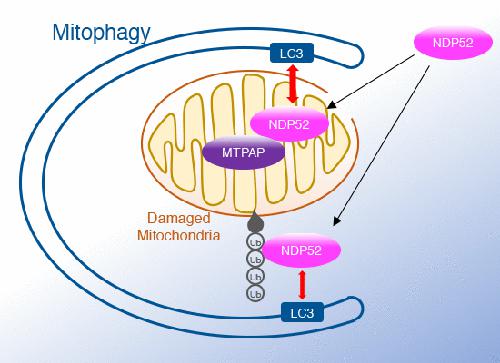
Parkin‐mediated mitophagy is a quality control pathway that selectively removes damaged mitochondria via the autophagic machinery. Autophagic receptors, which interact with ubiquitin and Atg8 family proteins, contribute to the recognition of damaged mitochondria by autophagosomes. NDP52, an autophagy receptor, is required for autophagic engulfment of damaged mitochondria during mitochondrial uncoupler treatment. The N‐terminal SKICH domain and C‐terminal zinc finger motif of NDP52 are both required for its function in mitophagy. While the zinc finger motif contributes to poly‐ubiquitin binding, the function of the SKICH domain remains unclear. Here, we show that NDP52 interacts with mitochondrial RNA poly(A) polymerase (MTPAP) via the SKICH domain. During mitophagy, NDP52 invades depolarized mitochondria and interacts with MTPAP dependent on the proteasome but independent of ubiquitin binding. Loss of MTPAP reduces NDP52‐mediated mitophagy, and the NDP52–MTPAP complex attracts more LC3 than NDP52 alone. These results indicate that NDP52 and MTPAP form an autophagy receptor complex, which enhances autophagic elimination of damaged mitochondria.
中文翻译:

NDP52与线粒体RNA poly(A)聚合酶相互作用以促进线粒体
Parkin介导的线粒体吞噬是一种质量控制途径,可通过自噬机制选择性去除受损的线粒体。自噬受体与泛素和Atg8家族蛋白相互作用,有助于自噬体识别受损的线粒体。在线粒体解偶联剂治疗期间,自噬吞噬受损的线粒体需要NDP 52,即自噬受体。NDP 52的N末端SKICH结构域和C末端锌指基序均是其在线粒体吞噬中的功能所必需的。尽管锌指基序有助于多泛素结合,但SKICH结构域的功能仍不清楚。在这里,我们显示NDP 52与线粒体相互作用通过SKICH域的RNA聚(A)聚合酶(MTPAP)。在线粒体吞噬过程中,NDP 52侵入去极化的线粒体并与MTPAP相互作用,依赖于蛋白酶体,但不依赖于泛素结合。损失MTPAP降低NDP 52介导的自噬和NDP 52- MTPAP复杂吸引更多的LC 3比NDP 52独自一人。这些结果表明,NDP 52和MTPAP形成自噬受体复合物,可增强自噬消除受损线粒体的能力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号