Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Promiscuous attraction of ligands within the ATP binding site of RyR2 promotes diverse gating behaviour.
Scientific Reports ( IF 3.8 ) Pub Date : 2018-Oct-09 , DOI: 10.1038/s41598-018-33328-8
Chris Lindsay , Mano Sitsapesan , Wei Mun Chan , Elisa Venturi , William Welch , Maria Musgaard , Rebecca Sitsapesan
Scientific Reports ( IF 3.8 ) Pub Date : 2018-Oct-09 , DOI: 10.1038/s41598-018-33328-8
Chris Lindsay , Mano Sitsapesan , Wei Mun Chan , Elisa Venturi , William Welch , Maria Musgaard , Rebecca Sitsapesan
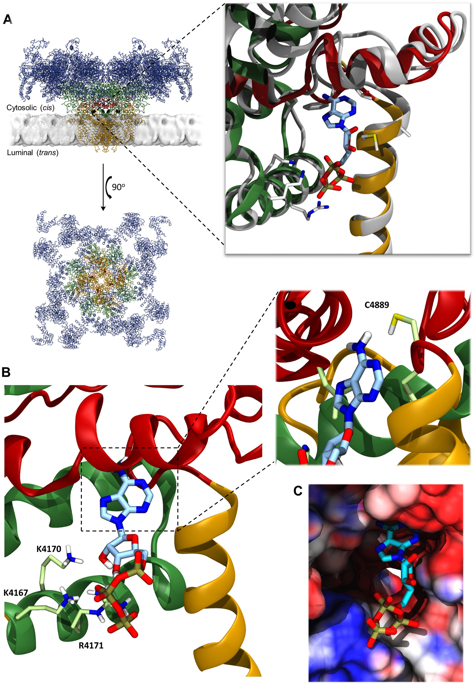
|
ATP is an essential constitutive regulator of cardiac ryanodine receptors (RyR2), enabling small changes in cytosolic Ca2+ to trigger large changes in channel activity. With recent landmark determinations of the full structures of RyR1 (skeletal isoform) and RyR2 using cryo-EM, and identification of the RyR1 ATP binding site, we have taken the opportunity to model the binding of fragments of ATP into RyR2 in order to investigate how the structure of the ATP site dictates the functional responses of ligands attracted there. RyR2 channel gating was assessed under voltage-clamp conditions and by [3H]ryanodine binding studies. We show that even the triphosphate (PPPi) moiety alone was capable of activating RyR2 but produced two distinct effects (activation or irreversible inactivation) that we suggest correspond to two preferred binding locations within the ATP site. Combinations of complementary fragments of ATP (Pi + ADP or PPi + AMP) could not reproduce the effects of ATP, however, the presence of adenosine prevented the inactivating PPPi effects, allowing activation similar to that of ATP. RyR2 appears to accommodate diverse types of molecules, including PPPi, deep within the ATP binding site. The most effective ligands, however, have at least three phosphate groups that are guided into place by a nucleoside.
中文翻译:

RyR2的ATP结合位点内的配体混杂吸引促进了多种门控行为。
ATP是心脏ryanodine受体(RyR2)的基本组成型调节剂,可使胞质Ca 2+中的微小变化触发通道活性的较大变化。最近使用冷冻EM对RyR1(骨骼亚型)和RyR2的完整结构进行了划时代的确定,并鉴定了RyR1 ATP结合位点,我们借此机会对ATP片段与RyR2的结合进行建模,以研究如何ATP位点的结构决定了吸引在那里的配体的功能响应。RyR2通道门控在电压钳制条件下通过[ 3H] ryanodine结合研究。我们显示,即使是单独的三磷酸酯(PPPi)部分也能够激活RyR2,但产生了两个不同的作用(激活或不可逆失活),我们建议对应于ATP位点中的两个首选结合位置。ATP互补片段(Pi + ADP或PPi + AMP)的组合不能重现ATP的作用,但是,腺苷的存在阻止了PPPi的失活,从而使活化作用类似于ATP。RyR2似乎可容纳ATP结合位点深处的各种类型的分子,包括PPPi。然而,最有效的配体具有至少三个被核苷引导到位的磷酸基团。
更新日期:2018-10-09
中文翻译:

RyR2的ATP结合位点内的配体混杂吸引促进了多种门控行为。
ATP是心脏ryanodine受体(RyR2)的基本组成型调节剂,可使胞质Ca 2+中的微小变化触发通道活性的较大变化。最近使用冷冻EM对RyR1(骨骼亚型)和RyR2的完整结构进行了划时代的确定,并鉴定了RyR1 ATP结合位点,我们借此机会对ATP片段与RyR2的结合进行建模,以研究如何ATP位点的结构决定了吸引在那里的配体的功能响应。RyR2通道门控在电压钳制条件下通过[ 3H] ryanodine结合研究。我们显示,即使是单独的三磷酸酯(PPPi)部分也能够激活RyR2,但产生了两个不同的作用(激活或不可逆失活),我们建议对应于ATP位点中的两个首选结合位置。ATP互补片段(Pi + ADP或PPi + AMP)的组合不能重现ATP的作用,但是,腺苷的存在阻止了PPPi的失活,从而使活化作用类似于ATP。RyR2似乎可容纳ATP结合位点深处的各种类型的分子,包括PPPi。然而,最有效的配体具有至少三个被核苷引导到位的磷酸基团。

































 京公网安备 11010802027423号
京公网安备 11010802027423号