当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen Activation Switch in the Copper Amine Oxidase of Escherichia coli.
Biochemistry ( IF 2.9 ) Pub Date : 2018-08-24 , DOI: 10.1021/acs.biochem.8b00633 Thembaninkosi G Gaule 1 , Mark A Smith 1 , Katarzyna M Tych 1, 2 , Pascale Pirrat 1 , Chi H Trinh 1 , Arwen R Pearson 1, 3 , Peter F Knowles 1 , Michael J McPherson 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-08-24 , DOI: 10.1021/acs.biochem.8b00633 Thembaninkosi G Gaule 1 , Mark A Smith 1 , Katarzyna M Tych 1, 2 , Pascale Pirrat 1 , Chi H Trinh 1 , Arwen R Pearson 1, 3 , Peter F Knowles 1 , Michael J McPherson 1
Affiliation
|
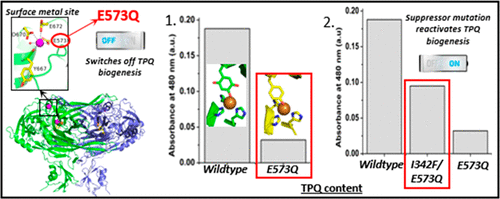
Copper amine oxidases (CuAOs) are metalloenzymes that reduce molecular oxygen to hydrogen peroxide during catalytic turnover of primary amines. In addition to Cu2+ in the active site, two peripheral calcium sites, ∼32 Å from the active site, have roles in Escherichia coli amine oxidase (ECAO). The buried Ca2+ (Asp533, Leu534, Asp535, Asp678, and Ala679) is essential for full-length protein production, while the surface Ca2+ (Glu573, Tyr667, Asp670, and Glu672) modulates biogenesis of the 2,4,5-trihydroxyphenylalanine quinone (TPQ) cofactor. The E573Q mutation at the surface site prevents calcium binding and TPQ biogenesis. However, TPQ biogenesis can be restored by a suppressor mutation (I342F) in the proposed oxygen delivery channel to the active site. While supporting TPQ biogenesis (∼60% WTECAO TPQ), I342F/E573Q has almost no amine oxidase activity (∼4.6% WTECAO activity). To understand how these long-range mutations have major effects on TPQ biogenesis and catalysis, we employed ultraviolet-visible spectroscopy, steady-state kinetics, inhibition assays, and X-ray crystallography. We show that the surface metal site controls the equilibrium (disproportionation) of the Cu2+-substrate reduced TPQ (TPQAMQ) Cu+-TPQ semiquinone (TPQSQ) couple. Removal of the calcium ion from this site by chelation or mutagenesis shifts the equilibrium to Cu2+-TPQAMQ or destabilizes Cu+-TPQSQ. Crystal structure analysis shows that TPQ biogenesis is stalled at deprotonation in the Cu2+-tyrosinate state. Our findings support WTECAO using the inner sphere electron transfer mechanism for oxygen reduction during catalysis, and while a Cu+-tyrosyl radical intermediate is not essential for TPQ biogenesis, it is required for efficient biogenesis.
中文翻译:

大肠杆菌铜胺氧化酶中的氧激活开关。
铜胺氧化酶 (CuAO) 是一种金属酶,可在伯胺的催化周转过程中将分子氧还原为过氧化氢。除了活性位点中的 Cu2+ 外,距离活性位点约 32 Å 的两个外围钙位点在大肠杆菌胺氧化酶 (ECAO) 中发挥作用。埋藏的 Ca2+(Asp533、Leu534、Asp535、Asp678 和 Ala679)对于全长蛋白质生产至关重要,而表面 Ca2+(Glu573、Tyr667、Asp670 和 Glu672)调节 2,4,5-三羟基苯丙氨酸醌的生物合成(TPQ) 辅因子。表面位点的 E573Q 突变阻止钙结合和 TPQ 生物发生。然而,TPQ 的生物发生可以通过提议的氧气输送通道中的抑制突变 (I342F) 恢复到活性位点。在支持 TPQ 生物发生的同时(~60% WTECAO TPQ),I342F/E573Q 几乎没有胺氧化酶活性(~4.6% WTECAO 活性)。为了了解这些远程突变如何对 TPQ 生物发生和催化产生重大影响,我们采用了紫外可见光谱、稳态动力学、抑制测定和 X 射线晶体学。我们表明,表面金属位点控制着 Cu2+-底物还原的 TPQ (TPQAMQ) Cu+-TPQ 半醌 (TPQSQ) 对的平衡(歧化)。通过螯合或诱变从该位点去除钙离子将平衡转移到 Cu2+-TPQAMQ 或使 Cu+-TPQSQ 不稳定。晶体结构分析表明,TPQ 的生物发生在 Cu2+-酪氨酸状态下的去质子化过程中停滞不前。我们的研究结果支持 WTECAO 在催化过程中使用内球电子转移机制进行氧还原,
更新日期:2018-08-15
中文翻译:

大肠杆菌铜胺氧化酶中的氧激活开关。
铜胺氧化酶 (CuAO) 是一种金属酶,可在伯胺的催化周转过程中将分子氧还原为过氧化氢。除了活性位点中的 Cu2+ 外,距离活性位点约 32 Å 的两个外围钙位点在大肠杆菌胺氧化酶 (ECAO) 中发挥作用。埋藏的 Ca2+(Asp533、Leu534、Asp535、Asp678 和 Ala679)对于全长蛋白质生产至关重要,而表面 Ca2+(Glu573、Tyr667、Asp670 和 Glu672)调节 2,4,5-三羟基苯丙氨酸醌的生物合成(TPQ) 辅因子。表面位点的 E573Q 突变阻止钙结合和 TPQ 生物发生。然而,TPQ 的生物发生可以通过提议的氧气输送通道中的抑制突变 (I342F) 恢复到活性位点。在支持 TPQ 生物发生的同时(~60% WTECAO TPQ),I342F/E573Q 几乎没有胺氧化酶活性(~4.6% WTECAO 活性)。为了了解这些远程突变如何对 TPQ 生物发生和催化产生重大影响,我们采用了紫外可见光谱、稳态动力学、抑制测定和 X 射线晶体学。我们表明,表面金属位点控制着 Cu2+-底物还原的 TPQ (TPQAMQ) Cu+-TPQ 半醌 (TPQSQ) 对的平衡(歧化)。通过螯合或诱变从该位点去除钙离子将平衡转移到 Cu2+-TPQAMQ 或使 Cu+-TPQSQ 不稳定。晶体结构分析表明,TPQ 的生物发生在 Cu2+-酪氨酸状态下的去质子化过程中停滞不前。我们的研究结果支持 WTECAO 在催化过程中使用内球电子转移机制进行氧还原,




















































 京公网安备 11010802027423号
京公网安备 11010802027423号