当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparing Protonolysis and Transmetalation Reactions: Microcalorimetric Studies of C–AuI Bonds in [AuRL] Complexes
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-08-14 00:00:00 , DOI: 10.1021/acs.inorgchem.8b01758
María Pérez-Iglesias 1 , Pablo Espinet 1 , Juan A. Casares 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-08-14 00:00:00 , DOI: 10.1021/acs.inorgchem.8b01758
María Pérez-Iglesias 1 , Pablo Espinet 1 , Juan A. Casares 1
Affiliation
|
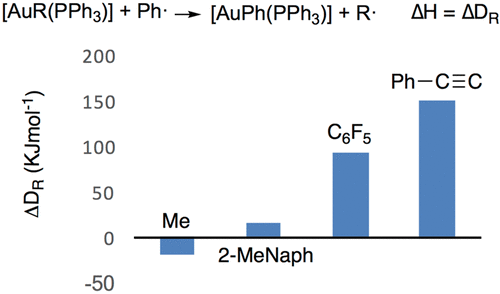
The protonolysis of C–Au bonds in [AuRL] organometallic complexes has been studied by calorimetry for 12 R groups. The experimental data have been combined with density functional theory calculations to obtain bond dissociation energy (BDE) values. The C–Au BDE values show a good correlation with the corresponding isolobal C–H BDE values. The heat released in the protonolysis of [AuRL] has also been measured for R = Ph and L = P(OPh)3, PPh3, PMe3, PCy3, and IPr, and these values strongly depend on the trans influence of L because of the mutual destabilization of the L–Au and Au–C bonds. The enthalpies of the transmetalation reaction [AuR(PPh3)] + SnIBu3 → [AuI(PPh3)] + SnRBu3 for seven R groups have been measured and compared with those of the corresponding [AuR(PPh3)] protonolysis.
中文翻译:

比较质子分解和金属转移反应:[AuRL]络合物中的C–Au I键的量热研究
通过量热法研究了12个R基团在[AuRL]有机金属配合物中C–Au键的质子分解。实验数据已与密度泛函理论计算相结合,以获得键离解能(BDE)值。C–Au BDE值与相应的等高C–H BDE值显示出良好的相关性。还测量了R = Ph和L = P(OPh)3,PPh 3,PMe 3,PCy 3和IPr在[AuRL]的质子分解过程中释放的热量,这些值在很大程度上取决于L的反式影响因为L–Au和Au–C键相互不稳定。过渡金属化反应的焓[AuR(PPh 3)] + SnIBu 3→[AUI(PPH 3)] + SnRBu 3七R基团已被测量并与那些相应的[AUR(PPH相比3)]质子分解。
更新日期:2018-08-14
中文翻译:

比较质子分解和金属转移反应:[AuRL]络合物中的C–Au I键的量热研究
通过量热法研究了12个R基团在[AuRL]有机金属配合物中C–Au键的质子分解。实验数据已与密度泛函理论计算相结合,以获得键离解能(BDE)值。C–Au BDE值与相应的等高C–H BDE值显示出良好的相关性。还测量了R = Ph和L = P(OPh)3,PPh 3,PMe 3,PCy 3和IPr在[AuRL]的质子分解过程中释放的热量,这些值在很大程度上取决于L的反式影响因为L–Au和Au–C键相互不稳定。过渡金属化反应的焓[AuR(PPh 3)] + SnIBu 3→[AUI(PPH 3)] + SnRBu 3七R基团已被测量并与那些相应的[AUR(PPH相比3)]质子分解。







































 京公网安备 11010802027423号
京公网安备 11010802027423号