当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anthracene‐Fused Dibenzo[def,mno]chrysenes with a Helical Structure
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-08-14 , DOI: 10.1002/ajoc.201800326
Qing Wang 1 , Gabriel Lim 1 , Tullimilli Y. Gopalakrishna 1 , Yi Han 1 , Chunyan Chi 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-08-14 , DOI: 10.1002/ajoc.201800326
Qing Wang 1 , Gabriel Lim 1 , Tullimilli Y. Gopalakrishna 1 , Yi Han 1 , Chunyan Chi 1
Affiliation
|
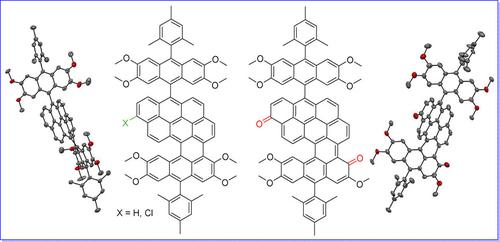
Scholl‐type oxidative cyclodehydrogenation reactions were conducted for an anthryl‐substituted dibenzo[def,mno]chrysene (DBC) precursor with the intention to prepare anthracene‐fused DBCs with extended conjugation. The compound ADBC‐1 with one cyclized ring and its two chloro‐ and oxy‐ derivatives (ADBC‐Cl and ADBC‐O) with good stability were successfully isolated and characterized. They showed largely red‐shifted absorption spectra and amphoteric redox behavior. XRD analysis revealed that they can be regarded as large polycyclic aromatic hydrocarbons (PAHs) containing [5]helicene substructures with large twist angles of 67.8°–73.7° owing to steric congestion. Further cyclization to form newer C−C bonds failed, which can be explained by an open‐shell diradical character and high HOMO energy levels of the products according to spin‐unrestricted DFT calculations.
中文翻译:

蒽融合的具有螺旋结构的二苯并[]
Scholl型氧化环脱氢反应是针对蒽基取代的二苯并[ def ] ,单价铬(DBC)前驱体进行的,目的是制备具有扩展共轭作用的蒽融合DBC。具有一个环的化合物ADBC-1及其两个氯和氧衍生物(ADBC-Cl和ADBC-O具有良好稳定性的)已成功分离和表征。它们显示出很大的红移吸收光谱和两性氧化还原行为。XRD分析表明,由于空间拥挤,它们可以被认为是含有[5]螺旋亚结构的大型多环芳烃(PAH),其扭曲角为67.8°–73.7°。根据自旋无限制DFT计算,进一步环化形成新的C-C键失败了,这可以用开壳双自由基特征和高HOMO能级来解释。
更新日期:2018-08-14
中文翻译:

蒽融合的具有螺旋结构的二苯并[]
Scholl型氧化环脱氢反应是针对蒽基取代的二苯并[ def ] ,单价铬(DBC)前驱体进行的,目的是制备具有扩展共轭作用的蒽融合DBC。具有一个环的化合物ADBC-1及其两个氯和氧衍生物(ADBC-Cl和ADBC-O具有良好稳定性的)已成功分离和表征。它们显示出很大的红移吸收光谱和两性氧化还原行为。XRD分析表明,由于空间拥挤,它们可以被认为是含有[5]螺旋亚结构的大型多环芳烃(PAH),其扭曲角为67.8°–73.7°。根据自旋无限制DFT计算,进一步环化形成新的C-C键失败了,这可以用开壳双自由基特征和高HOMO能级来解释。







































 京公网安备 11010802027423号
京公网安备 11010802027423号