当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics and Product Yields of the OH Initiated Oxidation of Hydroxymethyl Hydroperoxide
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1021/acs.jpca.8b04577 Hannah M. Allen , John D. Crounse , Kelvin H. Bates , Alexander Paichung Teng , Mitchell P. Krawiec-Thayer 1 , Jean C. Rivera-Rios 2 , Frank N. Keutsch 2 , Jason M. St. Clair 3, 4 , Thomas F. Hanisco 4 , Kristian H. Møller 5 , Henrik G. Kjaergaard 5 , Paul O. Wennberg
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1021/acs.jpca.8b04577 Hannah M. Allen , John D. Crounse , Kelvin H. Bates , Alexander Paichung Teng , Mitchell P. Krawiec-Thayer 1 , Jean C. Rivera-Rios 2 , Frank N. Keutsch 2 , Jason M. St. Clair 3, 4 , Thomas F. Hanisco 4 , Kristian H. Møller 5 , Henrik G. Kjaergaard 5 , Paul O. Wennberg
Affiliation
|
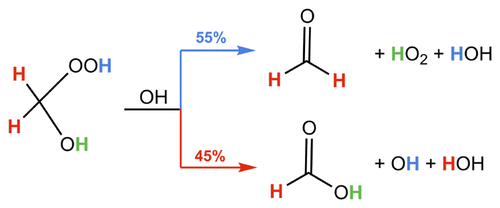
Hydroxymethyl hydroperoxide (HMHP), formed in the reaction of the C1 Criegee intermediate with water, is among the most abundant organic peroxides in the atmosphere. Although reaction with OH is thought to represent one of the most important atmospheric removal processes for HMHP, this reaction has been largely unstudied in the laboratory. Here, we present measurements of the kinetics and products formed in the reaction of HMHP with OH. HMHP was oxidized by OH in an environmental chamber; the decay of the hydroperoxide and the formation of formic acid and formaldehyde were monitored over time using CF3O– chemical ionization mass spectrometry (CIMS) and laser-induced fluorescence (LIF). The loss of HMHP by reaction with OH is measured relative to the loss of 1,2-butanediol [k1,2-butanediol+OH = (27.0 ± 5.6) × 10–12 cm3 molecule–1s–1]. We find that HMHP reacts with OH at 295 K with a rate coefficient of (7.1 ± 1.5) × 10–12 cm3 molecule–1s–1, with the formic acid to formaldehyde yield in a ratio of 0.88 ± 0.21 and independent of NO concentration (3 × 1010 – 1.5 × 1013 molecules cm–3). We suggest that, exclusively, abstraction of the methyl hydrogen of HMHP results in formic acid, while abstraction of the hydroperoxy hydrogen results in formaldehyde. We further evaluate the relative importance of HMHP sinks and use global simulations from GEOS-Chem to estimate that HMHP oxidation by OH contributes 1.7 Tg yr–1 (1–3%) of global annual formic acid production.
中文翻译:

OH引发的羟甲基过氧化氢的氧化动力学和产物收率
由C 1 Criegee中间体与水反应形成的羟甲基氢过氧化物(HMHP)是大气中最丰富的有机过氧化物之一。尽管与OH的反应被认为代表了HMHP最重要的大气去除方法之一,但该反应在实验室中尚未得到广泛研究。在这里,我们介绍了HMHP与OH反应过程中形成的动力学和产物的测量值。HMHP在环境室内被OH氧化; 使用CF 3 O –随时间监测氢过氧化物的降解以及甲酸和甲醛的形成化学电离质谱(CIMS)和激光诱导荧光(LIF)。相对于1,2-丁二醇的损失,测量了HMHP与OH反应的损失[ k 1,2-丁二醇+ OH =(27.0±5.6)×10 –12 cm 3分子–1 s –1 ]。我们发现,HMHP与295 K的OH反应,速率系数为(7.1±1.5)×10 –12 cm 3分子–1 s –1,甲酸与甲醛的产率为0.88±0.21,且与NO浓度(3×10 10 – 1.5×10 13分子cm –3)。我们建议,排他地,HMHP甲基氢的提取会导致甲酸,而氢过氧氢的提取会导致甲醛。我们进一步评估了HMHP水槽的相对重要性,并使用GEOS-Chem的全球模拟来估算OH引起的HMHP氧化占全球年甲酸产量的1.7 Tg yr –1(1-3%)。
更新日期:2018-07-11
中文翻译:

OH引发的羟甲基过氧化氢的氧化动力学和产物收率
由C 1 Criegee中间体与水反应形成的羟甲基氢过氧化物(HMHP)是大气中最丰富的有机过氧化物之一。尽管与OH的反应被认为代表了HMHP最重要的大气去除方法之一,但该反应在实验室中尚未得到广泛研究。在这里,我们介绍了HMHP与OH反应过程中形成的动力学和产物的测量值。HMHP在环境室内被OH氧化; 使用CF 3 O –随时间监测氢过氧化物的降解以及甲酸和甲醛的形成化学电离质谱(CIMS)和激光诱导荧光(LIF)。相对于1,2-丁二醇的损失,测量了HMHP与OH反应的损失[ k 1,2-丁二醇+ OH =(27.0±5.6)×10 –12 cm 3分子–1 s –1 ]。我们发现,HMHP与295 K的OH反应,速率系数为(7.1±1.5)×10 –12 cm 3分子–1 s –1,甲酸与甲醛的产率为0.88±0.21,且与NO浓度(3×10 10 – 1.5×10 13分子cm –3)。我们建议,排他地,HMHP甲基氢的提取会导致甲酸,而氢过氧氢的提取会导致甲醛。我们进一步评估了HMHP水槽的相对重要性,并使用GEOS-Chem的全球模拟来估算OH引起的HMHP氧化占全球年甲酸产量的1.7 Tg yr –1(1-3%)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号