当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamics of Mixing Primary Alkanolamines with Water
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-06-01 , DOI: 10.1021/acs.jpcb.8b01052
Abdenacer Idrissi 1 , Pál Jedlovszky 2
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-06-01 , DOI: 10.1021/acs.jpcb.8b01052
Abdenacer Idrissi 1 , Pál Jedlovszky 2
Affiliation
|
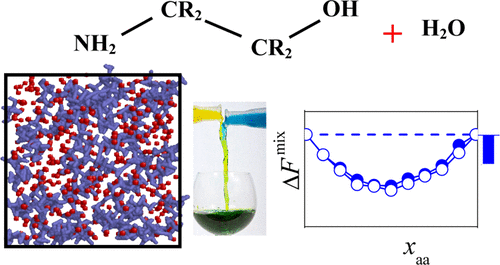
The volume, energy, entropy, and Helmholtz free energy of mixing of the seven simplest primary alkanolamine molecules, i.e., monoethanolamine, monoisopropanolamine, 2-amino-propan-1-ol, 2-amino-butan-1-ol, 2-amino-2-methyl-propan-1-ol, 1-amino-2-methyl-propan-2-ol, and 1-amino-butan-2-ol, with water is investigated by extensive computer simulations and thermodynamic integration. To check the force field dependence of the results, all calculations are repeated with two commonly used water models, namely, SPC/E and TIP4P. The obtained results show that the thermodynamics of mixing of alkanolamines and water is largely independent from the type of the alkanolamine molecule. The Helmholtz free energy of mixing is found to be negative for all alkanolamines at every composition, in accordance with the experimentally known full miscibility of these molecules and water. This free energy decrease occurring upon mixing is found to be clearly of energetic origin, as the energy of mixing always turns out to be negative in the entire composition range, while the entropy of mixing is also negative up to high alkanolamine mole fractions. The obtained results suggest that alkanolamines form, on average, stronger hydrogen bonds with water than what is formed by two water molecules, and they induce some ordering of the hydrating water molecules both through the hydrophobic hydration of their side chains and through the strong hydrogen bonding.
中文翻译:

伯烷醇胺与水混合的热力学
七个最简单的伯烷醇胺分子(即单乙醇胺,单异丙醇胺,2-氨基-丙-1-醇,2-氨基-丁-1-醇,2-氨基)的混合体积,能量,熵和亥姆霍兹自由能通过广泛的计算机模拟和热力学积分研究了-2-甲基-丙-1-醇,1-氨基-2-甲基-丙-2-醇和1-氨基-丁-2-醇与水的关系。为了检查结果对力场的依赖性,使用两个常用的水模型SPC / E和TIP4P重复了所有计算。所获得的结果表明,链烷醇胺与水混合的热力学在很大程度上与链烷醇胺分子的类型无关。发现在每种组成下,所有链烷醇胺的亥姆霍兹混合自由能均为负,根据实验已知这些分子与水的完全混溶性。发现混合时发生的这种自由能的减少显然是高能的,因为混合的能量在整个组成范围内总是变成负的,而混合的熵在高链烷醇胺摩尔分数下也是负的。所得结果表明,烷醇胺平均与水形成的氢键比由两个水分子形成的氢键强,并且它们通过侧链的疏水水合和强氢键诱导水合水分子的某些有序化。因为在整个组成范围内,混合能量始终为负,而在高链烷醇胺摩尔分数下,混合熵也为负。所得结果表明,烷醇胺平均与水形成的氢键比由两个水分子形成的氢键强,并且它们通过侧链的疏水水合和强氢键诱导水合水分子的某些有序化。因为在整个组成范围内,混合能量始终为负,而在高链烷醇胺摩尔分数下,混合熵也为负。所得结果表明,烷醇胺平均与水形成的氢键比由两个水分子形成的氢键强,并且它们通过侧链的疏水水合和强氢键诱导水合水分子的某些有序化。
更新日期:2018-06-01
中文翻译:

伯烷醇胺与水混合的热力学
七个最简单的伯烷醇胺分子(即单乙醇胺,单异丙醇胺,2-氨基-丙-1-醇,2-氨基-丁-1-醇,2-氨基)的混合体积,能量,熵和亥姆霍兹自由能通过广泛的计算机模拟和热力学积分研究了-2-甲基-丙-1-醇,1-氨基-2-甲基-丙-2-醇和1-氨基-丁-2-醇与水的关系。为了检查结果对力场的依赖性,使用两个常用的水模型SPC / E和TIP4P重复了所有计算。所获得的结果表明,链烷醇胺与水混合的热力学在很大程度上与链烷醇胺分子的类型无关。发现在每种组成下,所有链烷醇胺的亥姆霍兹混合自由能均为负,根据实验已知这些分子与水的完全混溶性。发现混合时发生的这种自由能的减少显然是高能的,因为混合的能量在整个组成范围内总是变成负的,而混合的熵在高链烷醇胺摩尔分数下也是负的。所得结果表明,烷醇胺平均与水形成的氢键比由两个水分子形成的氢键强,并且它们通过侧链的疏水水合和强氢键诱导水合水分子的某些有序化。因为在整个组成范围内,混合能量始终为负,而在高链烷醇胺摩尔分数下,混合熵也为负。所得结果表明,烷醇胺平均与水形成的氢键比由两个水分子形成的氢键强,并且它们通过侧链的疏水水合和强氢键诱导水合水分子的某些有序化。因为在整个组成范围内,混合能量始终为负,而在高链烷醇胺摩尔分数下,混合熵也为负。所得结果表明,烷醇胺平均与水形成的氢键比由两个水分子形成的氢键强,并且它们通过侧链的疏水水合和强氢键诱导水合水分子的某些有序化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号