当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Small Molecules Based on Alkyl/Alkylthio-thieno[3,2-b]thiophene-Substituted Benzo[1,2-b:4,5-b′]dithiophene for Solution-Processed Solar Cells with High Performance
Chemistry of Materials ( IF 7.2 ) Pub Date : 2015-11-30 00:00:00 , DOI: 10.1021/acs.chemmater.5b03889
Bin Kan , Qian Zhang , Feng Liu 1 , Xiangjian Wan , Yunchuang Wang , Wang Ni , Xuan Yang , Mingtao Zhang , Hongtao Zhang , Thomas P. Russell 1 , Yongsheng Chen
Chemistry of Materials ( IF 7.2 ) Pub Date : 2015-11-30 00:00:00 , DOI: 10.1021/acs.chemmater.5b03889
Bin Kan , Qian Zhang , Feng Liu 1 , Xiangjian Wan , Yunchuang Wang , Wang Ni , Xuan Yang , Mingtao Zhang , Hongtao Zhang , Thomas P. Russell 1 , Yongsheng Chen
Affiliation
|
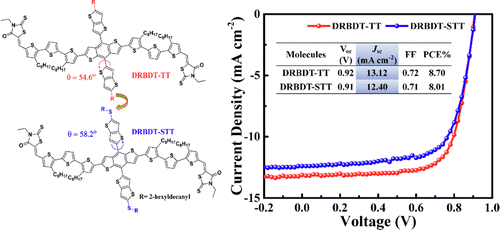
Two acceptor–donor–acceptor small molecules based on thieno[3,2-b]thiophene-substituted benzo[1,2-b:4,5-b′]dithiophene, DRBDT-TT with alkyl side chain and DRBDT-STT with alkylthio side chain, were designed and synthesized. Both molecules exhibit good thermal stability, suitable energy levels, and ordered molecular packing. Replacing the alkyl chain with alkylthio increases the dihedral angle between the thieno[3,2-b]thiophene (TT) and benzo[1,2-b:4,5-b′]dithiophene (BDT) unit, and thus slightly decreases its intermolecular interactions leading to its blue-shift absorption in the solid state. The best devices based on DRBDT-TT and DRBDT-STT both exhibited power conversion efficiencies (PCEs) over 8% with high fill factors (FFs) over 0.70 under AM 1.5G irradiation (100 mW cm–2), which are attributed to their optimized morphologies with feature size of 20–30 nm and well-balanced charge transport properties. The devices based on DRBDT-STT exhibited relatively lower short-circuit current density (Jsc) and thus slightly lower PCE as compared to the devices of DRBDT-TT, mainly due to its relatively poorer absorption. These results demonstrate that thieno[3,2-b]thiophene-substituted benzo[1,2-b:4,5-b′]dithiophene derivatives could be promising donor materials for obtaining high efficiencies and fill factors.
中文翻译:

小分子基于烷基/烷硫基-噻吩并[3,2- b ]噻吩取代的苯并[1,2 b:4,5-b']二对溶液处理的太阳能电池的高性能化
基于噻吩并[3,2 - b ]噻吩取代的苯并[1,2-b:4,5- b ']二噻吩的两个受体-供体-受体小分子,具有烷基侧链的DRBDT-TT和具有烷基侧链的DRBDT-STT设计并合成了烷硫基侧链。两种分子均显示出良好的热稳定性,合适的能级和有序的分子堆积。与烷硫基增加更换烷基链中的噻吩并[3,2-之间的二面角b ]噻吩(TT)和苯并[1,2-B:4,5- b']二噻吩(BDT)单元,因此略微降低其分子间相互作用,导致其在固态下发生蓝移吸收。基于DRBDT-TT和DRBDT-STT的最佳设备在AM 1.5G辐照(100 mW cm –2)下均表现出超过8%的功率转换效率(PCE)和超过0.70的高填充因子(FF)。具有20–30 nm的特征尺寸和均衡的电荷传输特性的优化形态。与DRBDT-TT的器件相比,基于DRBDT-STT的器件表现出相对较低的短路电流密度(J sc),因此PCE略低,这主要是由于其吸收性较差。这些结果表明噻吩并[3,2 - b ]噻吩取代的苯并[1,2-b:4,5-b ']二噻吩衍生物可能是有前途的供体材料,以获得高效率和填充因子。
更新日期:2015-11-30
中文翻译:

小分子基于烷基/烷硫基-噻吩并[3,2- b ]噻吩取代的苯并[1,2 b:4,5-b']二对溶液处理的太阳能电池的高性能化
基于噻吩并[3,2 - b ]噻吩取代的苯并[1,2-b:4,5- b ']二噻吩的两个受体-供体-受体小分子,具有烷基侧链的DRBDT-TT和具有烷基侧链的DRBDT-STT设计并合成了烷硫基侧链。两种分子均显示出良好的热稳定性,合适的能级和有序的分子堆积。与烷硫基增加更换烷基链中的噻吩并[3,2-之间的二面角b ]噻吩(TT)和苯并[1,2-B:4,5- b']二噻吩(BDT)单元,因此略微降低其分子间相互作用,导致其在固态下发生蓝移吸收。基于DRBDT-TT和DRBDT-STT的最佳设备在AM 1.5G辐照(100 mW cm –2)下均表现出超过8%的功率转换效率(PCE)和超过0.70的高填充因子(FF)。具有20–30 nm的特征尺寸和均衡的电荷传输特性的优化形态。与DRBDT-TT的器件相比,基于DRBDT-STT的器件表现出相对较低的短路电流密度(J sc),因此PCE略低,这主要是由于其吸收性较差。这些结果表明噻吩并[3,2 - b ]噻吩取代的苯并[1,2-b:4,5-b ']二噻吩衍生物可能是有前途的供体材料,以获得高效率和填充因子。








































 京公网安备 11010802027423号
京公网安备 11010802027423号