当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modification of alkyne-functionalized asymmetric phthalocyanines by CuI-catalyzed azide-alkyne cycloaddition
Tetrahedron ( IF 2.1 ) Pub Date : 2015-11-03 10:25:36 Xiaochun Chen, Chin-Wei Lu, Yiming Huang, Dominic V. McGrath
Tetrahedron ( IF 2.1 ) Pub Date : 2015-11-03 10:25:36 Xiaochun Chen, Chin-Wei Lu, Yiming Huang, Dominic V. McGrath
|
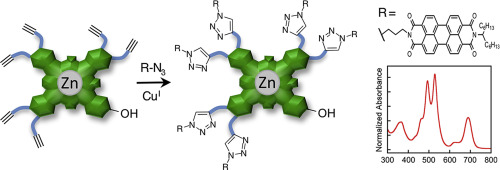
The use of asymmetric phthalocyanines (Pcs) as platforms for the preparation of several asymmetric hexatriazolyl-monohydroxyphthalocyanines via copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction was investigated. Asymmetric Pcs 5a and 5b were prepared through statistical macrocyclization of phthalonitriles (Pns) 1a and 2 to give PMB-protected 4a and 4b, which afforded asymmetric Pcs 5a and 5b after acidic cleavage. The ‘ROMP-Capture-Release’ method as a synthetic approach to prepare asymmetric Pc 5b was also evaluated. TIPS-protection of the terminal alkynes was necessary to prevent cross-coupling during the ring-opening metathesis polymerization (ROMP) step. Zinc Pc 5b was successfully used as a scaffold for functional modification under CuAAC conditions using several azides bearing hydrophobic, photo-crosslinkable, or electroactive moieties. Monitoring the CuAAC reaction by both UV/Vis and FTIR spectroscopies provided insight into the role of azide equivalent, reaction time, and catalyst on reaction progress.
中文翻译:

CuI催化的叠氮化物-炔烃环加成反应对炔烃官能化的不对称酞菁的改性
研究了使用不对称酞菁(Pcs)作为通过铜(I)催化的叠氮化物-炔烃环加成(CuAAC)反应制备几种不对称六三唑基-单羟基酞菁的平台。通过邻苯二甲腈(Pns)1a和2的统计大环化制备不对称的Pcs 5a和5b,得到PMB保护的4a和4b,在酸性裂解后得到不对称的Pcs 5a和5b。还评估了“ ROMP捕获释放”方法作为制备不对称Pc 5b的合成方法。末端炔烃的TIPS保护对于防止开环复分解聚合(ROMP)步骤期间的交叉偶联是必要的。Zc Pc 5b已成功用作CuAAC条件下的功能修饰支架,使用了几种带有疏水性,可光交联的叠氮化物,或电活性部分。通过UV / Vis和FTIR光谱监测CuAAC反应,可以深入了解叠氮化物当量,反应时间和催化剂对反应进程的作用。
更新日期:2015-11-04
中文翻译:

CuI催化的叠氮化物-炔烃环加成反应对炔烃官能化的不对称酞菁的改性
研究了使用不对称酞菁(Pcs)作为通过铜(I)催化的叠氮化物-炔烃环加成(CuAAC)反应制备几种不对称六三唑基-单羟基酞菁的平台。通过邻苯二甲腈(Pns)1a和2的统计大环化制备不对称的Pcs 5a和5b,得到PMB保护的4a和4b,在酸性裂解后得到不对称的Pcs 5a和5b。还评估了“ ROMP捕获释放”方法作为制备不对称Pc 5b的合成方法。末端炔烃的TIPS保护对于防止开环复分解聚合(ROMP)步骤期间的交叉偶联是必要的。Zc Pc 5b已成功用作CuAAC条件下的功能修饰支架,使用了几种带有疏水性,可光交联的叠氮化物,或电活性部分。通过UV / Vis和FTIR光谱监测CuAAC反应,可以深入了解叠氮化物当量,反应时间和催化剂对反应进程的作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号