当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heteroarylcarbene–Arylnitrene Radical Cation Isomerizations
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-02-21 00:00:00 , DOI: 10.1021/acs.jpca.9b00309
Didier Bégué 1 , Alain Dargelos 1 , Carl Braybrook 2 , Curt Wentrup 3
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-02-21 00:00:00 , DOI: 10.1021/acs.jpca.9b00309
Didier Bégué 1 , Alain Dargelos 1 , Carl Braybrook 2 , Curt Wentrup 3
Affiliation
|
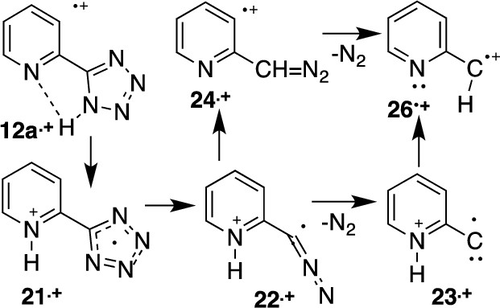
5-Phenyltetrazole 1e is an important source of phenylnitrene or the phenylnitrene radical cation (m/z 91) under thermal, photochemical, and electron impact conditions. Similarly, 3- or 4-(5-tetrazolyl)pyridines 12b,c yield pyridylnitrene radical cations 9a•+ (m/z 92) upon electron impact. In contrast, 2-(5-tetrazolyl)pyridine 12a•+ generates 2-pyridyldiazomethane 24•+ and 2-pyridylcarbene 26•+ radical cations (m/z 119 and 91) upon electron impact. The 2-pyridylcarbene radical cation undergoes a carbene–nitrene rearrangement to yield the phenylnitrene radical cation. Calculations at the B3LYP/6-311G(d,p) level have revealed facile H-transfer from the tetrazole to the pyridine ring in 2-(5-tetrazolyl)pyridine, 12a•+ → 21•+, taking place in the radical cations. Subsequent losses of N2 generate the pyridinium diazomethyl radical 22•+ or pyridinium-2-carbyne 23•+. These two ions can isomerize to 2-pyridyldiazomethane 24•+ and 2-pyridylcarbene 26•+, the latter rearranging to the phenylnitrene radical cations 9a•+. 13C-labeling of the tetrazole rings confirmed that 2-(5-tetrazolyl)pyridine 12a generates 2-pyridylcarbene/phenylnitrene radical cations retaining the 13C label, but 4-(5-tetrazolyl)pyridine 12c generates 4-pyridylnitrene 18c•+, which has lost the 13C label. 2-Pyridylcarbene/phenylnitrene radical cations (m/z 91) also constitute the base peak in the mass spectrum of 1,2,3-triazolo[1,5-a]pyridine 34. Similarly, 4-pyridylnitrene radical cation 18c•+ or its isomers (m/z 92) is obtained from 1,2,3-triazolo[1,5-a]pyrazine 36. Several other α-heteroaryltetrazoles behave in the same way as 2-(5-tetrazolyl)pyridine, yielding heteroarylcarbene/arylnitrene radical cations in the mass spectrometer, and this was confirmed by 13C-labeling in the case of 1-(5-tetrazolyl)isoquinoline 42-13C. In general, 5-aryltetrazoles generate arylnitrene radical cations under electron impact, but α-heteroaryltetrazoles generate α-heteroarylcarbene radical cations.
中文翻译:

杂芳基卡宾-芳基氮烯自由基阳离子异构化
5-苯基四唑1e在热,光化学和电子撞击条件下是苯硝烯或苯硝烯自由基阳离子(m / z 91)的重要来源。类似地,3-或4-(5-四唑基)吡啶12b,c在电子碰撞时产生吡啶基亚硝基苯甲酸根阳离子9a •+(m / z 92)。相反,2-(5-四唑基)吡啶12a •+生成2-吡啶基重氮甲烷24 •+和2-吡啶基卡宾26 •+自由基阳离子(m / z119和91)。2-吡啶基卡宾自由基阳离子经过卡宾-氮烯重排而生成苯基亚硝基自由基阳离子。在B3LYP / 6-311G(d,p)水平进行的计算表明,2-(5-四唑基)吡啶12a •+ → 21 •+中的四唑向吡啶环的氢容易转移,发生在自由基中阳离子。随后的N 2损失会生成吡啶鎓重氮甲基基团22 •+或吡啶-2-碳炔23 •+。这两个离子可以异构化为2-吡啶基重氮甲烷24 •+和2-吡啶基卡宾26 •+,后者重新排列为亚苯基亚硝基自由基阳离子9a •+。四唑环的13 C标记证实2-(5-四唑基)吡啶12a生成保留13 C标记的2-吡啶基碳烯/亚苯基吡啶阳离子,而4-(5-四唑基)吡啶12c生成4-吡啶基吡啶18c •+,它已经失去了13 C标签。2-吡啶基卡宾/亚苯基亚苯基自由基阳离子(m / z 91)在1,2,3-三唑并[1,5- a ]吡啶34的质谱图中也构成基本峰。同样,4-吡啶基亚硝基自由基阳离子18c•+或其异构体(m / z 92)从1,2,3-三唑并[1,5- a ]吡嗪36获得。其他几个α-杂芳基四唑的行为与2-(5-四唑基)吡啶相同,在质谱仪中产生杂芳基卡宾/芳基亚硝基自由基阳离子,在1-(5-四唑基)中通过13 C标记证实了这一点。)异喹啉42 - 13 ℃。在一般情况下,5-aryltetrazoles生成下电子撞击arylnitrene自由基阳离子,但α-heteroaryltetrazoles生成α-heteroarylcarbene自由基阳离子。
更新日期:2019-02-21
中文翻译:

杂芳基卡宾-芳基氮烯自由基阳离子异构化
5-苯基四唑1e在热,光化学和电子撞击条件下是苯硝烯或苯硝烯自由基阳离子(m / z 91)的重要来源。类似地,3-或4-(5-四唑基)吡啶12b,c在电子碰撞时产生吡啶基亚硝基苯甲酸根阳离子9a •+(m / z 92)。相反,2-(5-四唑基)吡啶12a •+生成2-吡啶基重氮甲烷24 •+和2-吡啶基卡宾26 •+自由基阳离子(m / z119和91)。2-吡啶基卡宾自由基阳离子经过卡宾-氮烯重排而生成苯基亚硝基自由基阳离子。在B3LYP / 6-311G(d,p)水平进行的计算表明,2-(5-四唑基)吡啶12a •+ → 21 •+中的四唑向吡啶环的氢容易转移,发生在自由基中阳离子。随后的N 2损失会生成吡啶鎓重氮甲基基团22 •+或吡啶-2-碳炔23 •+。这两个离子可以异构化为2-吡啶基重氮甲烷24 •+和2-吡啶基卡宾26 •+,后者重新排列为亚苯基亚硝基自由基阳离子9a •+。四唑环的13 C标记证实2-(5-四唑基)吡啶12a生成保留13 C标记的2-吡啶基碳烯/亚苯基吡啶阳离子,而4-(5-四唑基)吡啶12c生成4-吡啶基吡啶18c •+,它已经失去了13 C标签。2-吡啶基卡宾/亚苯基亚苯基自由基阳离子(m / z 91)在1,2,3-三唑并[1,5- a ]吡啶34的质谱图中也构成基本峰。同样,4-吡啶基亚硝基自由基阳离子18c•+或其异构体(m / z 92)从1,2,3-三唑并[1,5- a ]吡嗪36获得。其他几个α-杂芳基四唑的行为与2-(5-四唑基)吡啶相同,在质谱仪中产生杂芳基卡宾/芳基亚硝基自由基阳离子,在1-(5-四唑基)中通过13 C标记证实了这一点。)异喹啉42 - 13 ℃。在一般情况下,5-aryltetrazoles生成下电子撞击arylnitrene自由基阳离子,但α-heteroaryltetrazoles生成α-heteroarylcarbene自由基阳离子。




































 京公网安备 11010802027423号
京公网安备 11010802027423号