当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronic and Molecular Structures of the CeB6 Monomer
The Journal of Physical Chemistry A ( IF 2.8 ) Pub Date : 2019-02-21 00:00:00 , DOI: 10.1021/acs.jpca.8b12399
Jarrett L. Mason , Hassan Harb , Caleb D. Huizenga , Joshua C. Ewigleben , Josey E. Topolski , Hrant P. Hratchian , Caroline Chick Jarrold
The Journal of Physical Chemistry A ( IF 2.8 ) Pub Date : 2019-02-21 00:00:00 , DOI: 10.1021/acs.jpca.8b12399
Jarrett L. Mason , Hassan Harb , Caleb D. Huizenga , Joshua C. Ewigleben , Josey E. Topolski , Hrant P. Hratchian , Caroline Chick Jarrold
|
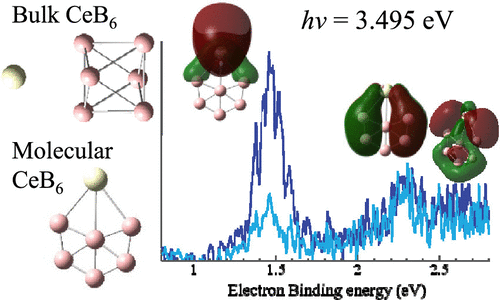
The electronic and molecular structure of the CeB6 molecular unit has been probed by anion PE spectroscopy and DFT calculations to gain insight into structural and electronic relaxation on edge and corner sites of this ionic material. While boron in bulk lanthanide hexaboride materials assumes octahedral B63– units, the monomer assumes a less compact structure to delocalize the charge. Two competitive molecular structures were identified for the anion and neutral species, which include a boat-like structure and a planar or near-planar teardrop structure. Ce adopts different orbital occupancies in the two isomers; the boat-like structure has a 4f superconfiguration while the teardrop favors a 4f 6s occupancy. The B6 ligand in these structures carries a charge of −4 and −3, respectively. The teardrop structure, which was calculated to be isoenergetic with the boat structure, was most consistent with the experimental spectrum. B6-local orbitals crowd the energy window between the Ce 4f and 6s (HOMO) orbitals. A low-lying transition from the B-based orbitals is observed slightly less than 1 eV above the ground state. The results suggest that edge and corner conductivity involves stabilized, highly diffuse 6s orbitals or bands rather than the bulk-favored 5d band. High-spin and open-shell low-spin states were calculated to be very close in energy for both the anion and neutral, a characteristic that reflects how decoupled the 4f electron is from the B6 2p- and Ce 6s-based molecular orbitals.
中文翻译:

CeB 6单体的电子和分子结构
CeB 6分子单元的电子和分子结构已通过阴离子PE光谱和DFT计算进行了探索,以深入了解这种离子材料的边缘和角落部位的结构和电子弛豫。块状镧系六硼化物材料中的硼假定为八面体B 6 3–单元,而单体则具有较不紧凑的结构以使电荷离域。对于阴离子和中性物质,鉴定了两个竞争性分子结构,包括船形结构和平面或近平面的泪滴结构。Ce在两种异构体中采用不同的轨道占有率;船形结构具有4f超级配置,而水滴则有利于4f 6s的占用。B 6这些结构中的配体分别带有-4和-3电荷。泪滴结构被计算为与船形结构等能量,与实验光谱最一致。B 6-局部轨道将能量窗口拥塞在Ce 4f和6s(HOMO)轨道之间。观察到来自B基轨道的低空跃迁略高于基态1 eV。结果表明,边缘和角部电导率涉及稳定的,高度扩散的6s轨道或能带,而不是整体偏爱的5d频带。计算得出,高旋转态和开壳式低旋转态的阴离子和中性能量非常接近,这一特征反映了4f电子与基于B 6 2p和Ce 6s的分子轨道之间的去耦关系。
更新日期:2019-02-21
中文翻译:

CeB 6单体的电子和分子结构
CeB 6分子单元的电子和分子结构已通过阴离子PE光谱和DFT计算进行了探索,以深入了解这种离子材料的边缘和角落部位的结构和电子弛豫。块状镧系六硼化物材料中的硼假定为八面体B 6 3–单元,而单体则具有较不紧凑的结构以使电荷离域。对于阴离子和中性物质,鉴定了两个竞争性分子结构,包括船形结构和平面或近平面的泪滴结构。Ce在两种异构体中采用不同的轨道占有率;船形结构具有4f超级配置,而水滴则有利于4f 6s的占用。B 6这些结构中的配体分别带有-4和-3电荷。泪滴结构被计算为与船形结构等能量,与实验光谱最一致。B 6-局部轨道将能量窗口拥塞在Ce 4f和6s(HOMO)轨道之间。观察到来自B基轨道的低空跃迁略高于基态1 eV。结果表明,边缘和角部电导率涉及稳定的,高度扩散的6s轨道或能带,而不是整体偏爱的5d频带。计算得出,高旋转态和开壳式低旋转态的阴离子和中性能量非常接近,这一特征反映了4f电子与基于B 6 2p和Ce 6s的分子轨道之间的去耦关系。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号