当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functionalized Corannulene Carbocations: A Structural Overview
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-07-28 , DOI: 10.1002/chem.201500697
Cristina Dubceac , Alexander S. Filatov , Alexander V. Zabula , Andrey Yu. Rogachev , Marina A. Petrukhina
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-07-28 , DOI: 10.1002/chem.201500697
Cristina Dubceac , Alexander S. Filatov , Alexander V. Zabula , Andrey Yu. Rogachev , Marina A. Petrukhina
|
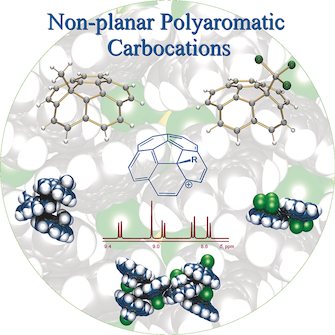
A detailed structural overview of a family of bowl‐shaped polycyclic aromatic carbocations of the type [C20H10R]+ with different R functionalities tethered to the interior surface of corannulene (C20H10) is provided. Changing the identity of the surface‐bound groups through alkyl chains spanning from one to four carbon atoms and incorporating a different degree of halogenation has led to the fine tuning of the bowl structures and properties. The deformation of the corannulene core upon functionalization has been revealed based on X‐ray crystallographic analysis and compared for the series of cations with R=CH3, CH2Cl, CHCl2, CCl3, CH2CH3, CH2CH2Cl, and CH2CH2Br. The resulting carbocations have been isolated with several metal‐based counterions, varying in size and coordinating abilities ([AlCl4]−, [AlBr4]−, [(SnCl)(GaCl4)2]−, and [Al(OC(CF3)3)4]−). A variety of aggregation patterns in the solid state has been revealed based on different intermolecular interactions ranging from cation–anion to π–π stacking and to halogen⋅⋅⋅π interactions. For the [C20H10CH2Cl]+ ion crystallized with several different counterions, the conformation of the R group attached to the central five‐membered ring of corannulene moiety was found to depend on the solid‐state environment defined by the identity of anions. Solution NMR and UV/Vis investigations have been used to complement the X‐ray diffraction studies for this series of corannulene‐based cations and to demonstrate their different association patterns with the solvent molecules.
中文翻译:

功能化的Corannulene碳正离子:结构概述
提供了一个碗形的[C 20 H 10 R] +型碗形多环芳族碳正离子的详细结构概述,其中,C环戊烯(C 20 H 10)的内表面具有不同的R官能度。通过跨越一个碳原子到四个碳原子的烷基链来改变表面结合基团的身份,并引入不同程度的卤化,导致对碗结构和性能的微调。通过X射线晶体学分析揭示了官能团中的氢化萘环核的形变,并与R = CH 3,CH 2 Cl,CHCl 2,CCl的一系列阳离子进行了比较3,CH 2 CH 3,CH 2 CH 2 Cl和CH 2 CH 2 Br。生成的碳正离子已通过几种基于金属的抗衡离子进行了分离,它们的大小和配位能力各不相同([AlCl 4 ] -,[AlBr 4 ] -,[(SnCl)(GaCl 4)2 ] -和[Al(OC( CF 3)3)4 ] -)。基于不同的分子间相互作用,从阳离子-阴离子到π-π堆积和卤素⋅⋅⋅π相互作用,揭示了固态的各种聚集模式。对于用几个不同的抗衡离子结晶的[C 20 H 10 CH 2 Cl] +离子,发现附接到环戊烯基部分中心五元环上的R基团的构型取决于身份所定义的固态环境阴离子。溶液NMR和UV / Vis研究已用于补充该系列基于环戊二烯的阳离子的X射线衍射研究,并证明了它们与溶剂分子的不同缔合方式。
更新日期:2015-07-28
中文翻译:

功能化的Corannulene碳正离子:结构概述
提供了一个碗形的[C 20 H 10 R] +型碗形多环芳族碳正离子的详细结构概述,其中,C环戊烯(C 20 H 10)的内表面具有不同的R官能度。通过跨越一个碳原子到四个碳原子的烷基链来改变表面结合基团的身份,并引入不同程度的卤化,导致对碗结构和性能的微调。通过X射线晶体学分析揭示了官能团中的氢化萘环核的形变,并与R = CH 3,CH 2 Cl,CHCl 2,CCl的一系列阳离子进行了比较3,CH 2 CH 3,CH 2 CH 2 Cl和CH 2 CH 2 Br。生成的碳正离子已通过几种基于金属的抗衡离子进行了分离,它们的大小和配位能力各不相同([AlCl 4 ] -,[AlBr 4 ] -,[(SnCl)(GaCl 4)2 ] -和[Al(OC( CF 3)3)4 ] -)。基于不同的分子间相互作用,从阳离子-阴离子到π-π堆积和卤素⋅⋅⋅π相互作用,揭示了固态的各种聚集模式。对于用几个不同的抗衡离子结晶的[C 20 H 10 CH 2 Cl] +离子,发现附接到环戊烯基部分中心五元环上的R基团的构型取决于身份所定义的固态环境阴离子。溶液NMR和UV / Vis研究已用于补充该系列基于环戊二烯的阳离子的X射线衍射研究,并证明了它们与溶剂分子的不同缔合方式。



































 京公网安备 11010802027423号
京公网安备 11010802027423号