当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unusually Stable Isoxazolidinyl Epoxides: Synthesis and Reactivity in Nucleophilic Substitutions
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2016-07-25 , DOI: 10.1002/ejoc.201600471 Ondrej Záborský 1 , Tomáš Malatinský 1 , Jaromír Marek 2 , Ján Moncol 3 , Róbert Fischer 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2016-07-25 , DOI: 10.1002/ejoc.201600471 Ondrej Záborský 1 , Tomáš Malatinský 1 , Jaromír Marek 2 , Ján Moncol 3 , Róbert Fischer 1
Affiliation
|
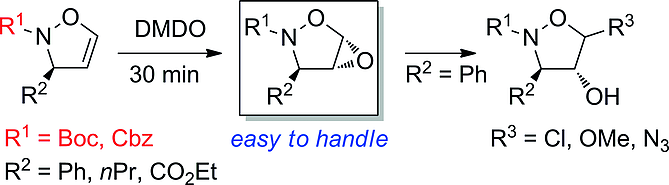
The synthesis of new N-carbamoyl-substituted isoxazolidine-4,5-diols and their 4,5-anhydro analogues, isoxazolidinyl epoxides, is described. The 4,5-unsubstituted 2,3-dihydroisoxazole starting materials reacted directly with potassium osmate/4-methylmorpholine N-oxide and 3,3-dimethyldioxirane, respectively. All addition reactions proceeded with excellent anti selectivity with respect to the substituent at C-3. The obtained isoxazolidinyl epoxides as well as benzoylated isoxazolidine-4,5-diols were subjected to nucleophilic substitution reactions with various nucleophiles. 4-Hydroxyisoxazolidines with chlorine, methoxy, and azide substituents bound to C-5 were prepared in moderate to good yields. In terms of chemical stability, the discussed isoxazolidinyl epoxides can be isolated and stored for a long time.
中文翻译:

异常稳定的异恶唑烷基环氧化物:亲核取代的合成和反应性
描述了新的 N-氨基甲酰基取代的异恶唑烷-4,5-二醇及其 4,5-脱水类似物异恶唑烷基环氧化物的合成。4,5-未取代的2,3-二氢异恶唑原料分别与锇酸钾/4-甲基吗啉N-氧化物和3,3-二甲基二环氧乙烷直接反应。所有加成反应均以对 C-3 处取代基的优异抗选择性进行。获得的异恶唑烷基环氧化物以及苯甲酰化的异恶唑烷-4,5-二醇与各种亲核试剂进行亲核取代反应。以中等至良好的产率制备了氯、甲氧基和叠氮化物取代基与 C-5 结合的 4-羟基异恶唑烷。在化学稳定性方面,所讨论的异恶唑烷基环氧化物可以分离并长期储存。
更新日期:2016-07-25
中文翻译:

异常稳定的异恶唑烷基环氧化物:亲核取代的合成和反应性
描述了新的 N-氨基甲酰基取代的异恶唑烷-4,5-二醇及其 4,5-脱水类似物异恶唑烷基环氧化物的合成。4,5-未取代的2,3-二氢异恶唑原料分别与锇酸钾/4-甲基吗啉N-氧化物和3,3-二甲基二环氧乙烷直接反应。所有加成反应均以对 C-3 处取代基的优异抗选择性进行。获得的异恶唑烷基环氧化物以及苯甲酰化的异恶唑烷-4,5-二醇与各种亲核试剂进行亲核取代反应。以中等至良好的产率制备了氯、甲氧基和叠氮化物取代基与 C-5 结合的 4-羟基异恶唑烷。在化学稳定性方面,所讨论的异恶唑烷基环氧化物可以分离并长期储存。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号