当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational heterogeneity of the calmodulin binding interface.
Nature Communications ( IF 14.7 ) Pub Date : 2016-Apr-04 , DOI: 10.1038/ncomms10910 Diwakar Shukla 1, 2, 3 , Ariana Peck 4 , Vijay S Pande 1, 2
Nature Communications ( IF 14.7 ) Pub Date : 2016-Apr-04 , DOI: 10.1038/ncomms10910 Diwakar Shukla 1, 2, 3 , Ariana Peck 4 , Vijay S Pande 1, 2
Affiliation
|
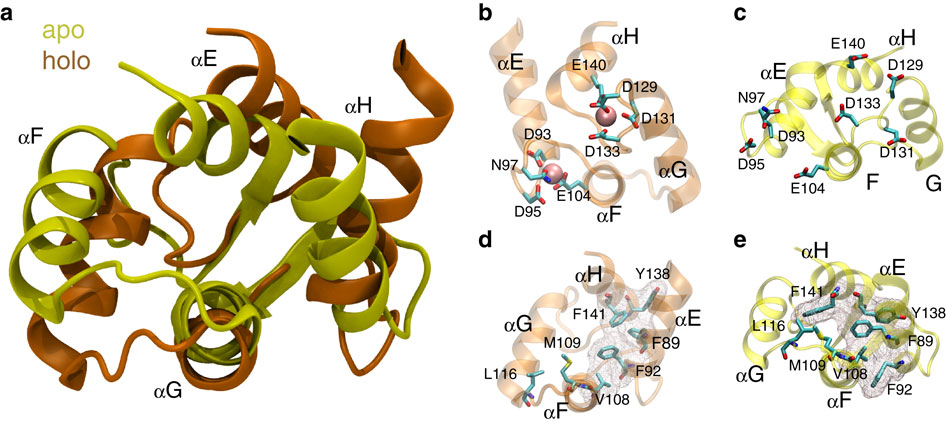
Calmodulin (CaM) is a ubiquitous Ca(2+) sensor and a crucial signalling hub in many pathways aberrantly activated in disease. However, the mechanistic basis of its ability to bind diverse signalling molecules including G-protein-coupled receptors, ion channels and kinases remains poorly understood. Here we harness the high resolution of molecular dynamics simulations and the analytical power of Markov state models to dissect the molecular underpinnings of CaM binding diversity. Our computational model indicates that in the absence of Ca(2+), sub-states in the folded ensemble of CaM's C-terminal domain present chemically and sterically distinct topologies that may facilitate conformational selection. Furthermore, we find that local unfolding is off-pathway for the exchange process relevant for peptide binding, in contrast to prior hypotheses that unfolding might account for binding diversity. Finally, our model predicts a novel binding interface that is well-populated in the Ca(2+)-bound regime and, thus, a candidate for pharmacological intervention.
中文翻译:

钙调蛋白结合界面的构象异质性。
钙调蛋白 (CaM) 是一种普遍存在的 Ca(2+) 传感器,也是疾病中异常激活的许多通路中的关键信号中枢。然而,其结合多种信号分子(包括 G 蛋白偶联受体、离子通道和激酶)能力的机制基础仍然知之甚少。在这里,我们利用分子动力学模拟的高分辨率和马尔可夫状态模型的分析能力来剖析 CaM 结合多样性的分子基础。我们的计算模型表明,在没有 Ca(2+) 的情况下,CaM C 端结构域折叠整体中的亚态呈现出化学和空间上不同的拓扑,这可能有助于构象选择。此外,我们发现局部解折叠对于与肽结合相关的交换过程是偏离路径的,这与解折叠可能解释结合多样性的先前假设相反。最后,我们的模型预测了一种新的结合界面,该界面在 Ca(2+) 结合区域中分布广泛,因此是药物干预的候选者。
更新日期:2016-04-07
中文翻译:

钙调蛋白结合界面的构象异质性。
钙调蛋白 (CaM) 是一种普遍存在的 Ca(2+) 传感器,也是疾病中异常激活的许多通路中的关键信号中枢。然而,其结合多种信号分子(包括 G 蛋白偶联受体、离子通道和激酶)能力的机制基础仍然知之甚少。在这里,我们利用分子动力学模拟的高分辨率和马尔可夫状态模型的分析能力来剖析 CaM 结合多样性的分子基础。我们的计算模型表明,在没有 Ca(2+) 的情况下,CaM C 端结构域折叠整体中的亚态呈现出化学和空间上不同的拓扑,这可能有助于构象选择。此外,我们发现局部解折叠对于与肽结合相关的交换过程是偏离路径的,这与解折叠可能解释结合多样性的先前假设相反。最后,我们的模型预测了一种新的结合界面,该界面在 Ca(2+) 结合区域中分布广泛,因此是药物干预的候选者。





























 京公网安备 11010802027423号
京公网安备 11010802027423号