当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ni–M–O (M = Sn, Ti, W) Catalysts Prepared by a Dry Mixing Method for Oxidative Dehydrogenation of Ethane
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-04-05 00:00:00 , DOI: 10.1021/acscatal.6b00044
Haibo Zhu 1 , Devon C. Rosenfeld 2 , Moussab Harb 1 , Dalaver H. Anjum 3 , Mohamed Nejib Hedhili 3 , Samy Ould-Chikh 1 , Jean-Marie Basset 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-04-05 00:00:00 , DOI: 10.1021/acscatal.6b00044
Haibo Zhu 1 , Devon C. Rosenfeld 2 , Moussab Harb 1 , Dalaver H. Anjum 3 , Mohamed Nejib Hedhili 3 , Samy Ould-Chikh 1 , Jean-Marie Basset 1
Affiliation
|
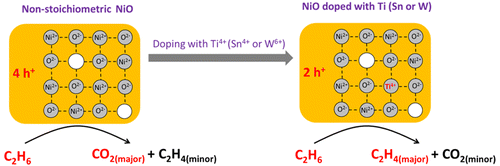
A new generation of Ni–Sn–O, Ni–Ti–O, and Ni–W–O catalysts has been prepared by a solid-state grinding method. In each case the doping metal varied from 2.5% to 20%. These catalysts exhibited higher activity and selectivity for ethane oxidative dehydrogenation (ODH) than conventionally prepared mixed oxides. Detailed characterization was achieved using XRD, N2 adsorption, H2-TPR, SEM, TEM, and HAADF-STEM in order to study the detailed atomic structure and textural properties of the synthesized catalysts. Two kinds of typical structures are found in these mixed oxides, which are (major) “NixMyO” (M = Sn, Ti, W) solid solution phases (NiO crystalline structure with doping atom incorporated in the lattice) and (minor) secondary phases (SnO2, TiO2, or WO3). The secondary phase exists as a thin layer around small “NixMyO” particles, lowering the aggregation of nanoparticles during the synthesis. DFT calculations on the formation energies of M-doped NiO structures (M = Sn, Ti, W) clearly confirm the thermodynamic feasibility of incorporating these doping metals into the NiO struture. The incorporation of doping metals into the NiO lattice decreases the number of holes (h+) localized on lattice oxygen (O2– + h+ → O•–) , which is the main reason for the improved catalytic performance (O•– is known to favor complete ethane oxidation to CO2). The high efficiency of ethylene production achieved in these particularly prepared mixed oxide catalysts indicates that the solid grinding method could serve as a general and practical approach for the preparation of doped NiO-based catalysts.
中文翻译:

干混合法制备的乙烷氧化脱氢的Ni–M–O(M = Sn,Ti,W)催化剂
通过固态研磨方法已经制备了新一代的Ni–Sn–O,Ni–Ti–O和Ni–W–O催化剂。在每种情况下,掺杂金属的含量从2.5%到20%不等。与常规制备的混合氧化物相比,这些催化剂对乙烷氧化脱氢(ODH)表现出更高的活性和选择性。为了研究合成催化剂的详细原子结构和结构性质,使用XRD,N 2吸附,H 2 -TPR,SEM,TEM和HAADF-STEM进行了详细的表征。在这些混合氧化物中发现两种典型的结构,即(主要)“ Ni x M yO”(M = Sn,Ti,W)固溶相(在晶格中掺入掺杂原子的NiO晶体结构)和(次要)次级相(SnO 2,TiO 2或WO 3)。第二相以薄的“ Ni x M y O”颗粒周围的薄层存在,降低了合成过程中纳米颗粒的聚集。D掺杂M掺杂NiO结构(M = Sn,Ti,W)形成能的DFT计算清楚地证实了将这些掺杂金属掺入NiO结构的热力学可行性。在NiO晶格中掺入掺杂金属会减少位于晶格氧(O 2– + h + →O )上的空穴(h +)的数量•–),这是提高催化性能的主要原因(众所周知,O •–有利于将乙烷完全氧化为CO 2)。在这些特别制备的混合氧化物催化剂中实现的高乙烯生产效率表明,固体研磨方法可作为制备掺杂的NiO基催化剂的通用方法。
更新日期:2016-04-05
中文翻译:

干混合法制备的乙烷氧化脱氢的Ni–M–O(M = Sn,Ti,W)催化剂
通过固态研磨方法已经制备了新一代的Ni–Sn–O,Ni–Ti–O和Ni–W–O催化剂。在每种情况下,掺杂金属的含量从2.5%到20%不等。与常规制备的混合氧化物相比,这些催化剂对乙烷氧化脱氢(ODH)表现出更高的活性和选择性。为了研究合成催化剂的详细原子结构和结构性质,使用XRD,N 2吸附,H 2 -TPR,SEM,TEM和HAADF-STEM进行了详细的表征。在这些混合氧化物中发现两种典型的结构,即(主要)“ Ni x M yO”(M = Sn,Ti,W)固溶相(在晶格中掺入掺杂原子的NiO晶体结构)和(次要)次级相(SnO 2,TiO 2或WO 3)。第二相以薄的“ Ni x M y O”颗粒周围的薄层存在,降低了合成过程中纳米颗粒的聚集。D掺杂M掺杂NiO结构(M = Sn,Ti,W)形成能的DFT计算清楚地证实了将这些掺杂金属掺入NiO结构的热力学可行性。在NiO晶格中掺入掺杂金属会减少位于晶格氧(O 2– + h + →O )上的空穴(h +)的数量•–),这是提高催化性能的主要原因(众所周知,O •–有利于将乙烷完全氧化为CO 2)。在这些特别制备的混合氧化物催化剂中实现的高乙烯生产效率表明,固体研磨方法可作为制备掺杂的NiO基催化剂的通用方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号