当前位置:
X-MOL 学术
›
JAMA Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Derivation of Anthracycline and Anthraquinone Equivalence Ratios to Doxorubicin for Late-Onset Cardiotoxicity.
JAMA Oncology ( IF 22.5 ) Pub Date : 2019-06-01 , DOI: 10.1001/jamaoncol.2018.6634
Elizabeth A M Feijen 1, 2 , Wendy M Leisenring 3, 4 , Kayla L Stratton 3, 4 , Kirsten K Ness 5 , Helena J H van der Pal 2 , Elvira C van Dalen 1, 2 , Gregory T Armstrong 5 , Gregory J Aune 6 , Daniel M Green 5 , Melissa M Hudson 7 , Jacqueline Loonen 8 , Kevin C Oeffinger 9 , Leslie L Robison 5 , Yutaka Yasui 5 , Leontien C M Kremer 1, 2 , Eric J Chow 3, 4, 10
JAMA Oncology ( IF 22.5 ) Pub Date : 2019-06-01 , DOI: 10.1001/jamaoncol.2018.6634
Elizabeth A M Feijen 1, 2 , Wendy M Leisenring 3, 4 , Kayla L Stratton 3, 4 , Kirsten K Ness 5 , Helena J H van der Pal 2 , Elvira C van Dalen 1, 2 , Gregory T Armstrong 5 , Gregory J Aune 6 , Daniel M Green 5 , Melissa M Hudson 7 , Jacqueline Loonen 8 , Kevin C Oeffinger 9 , Leslie L Robison 5 , Yutaka Yasui 5 , Leontien C M Kremer 1, 2 , Eric J Chow 3, 4, 10
Affiliation
|
Importance
Anthracyclines are part of many effective pediatric cancer treatment protocols. Most pediatric oncology treatment groups assume that the hematologic toxicity of anthracycline agents is equivalent to their cardiotoxicity; for example, Children's Oncology Group substitution rules consider daunorubicin and epirubicin isoequivalent to doxorubicin, whereas mitoxantrone and idarubicin are considered 4 to 5 times as toxic as doxorubicin.
Objective
To determine optimal dose equivalence ratios for late-onset cardiomyopathy between doxorubicin and other anthracyclines or the anthraquinone mitoxantrone.
Design, Setting, and Participants
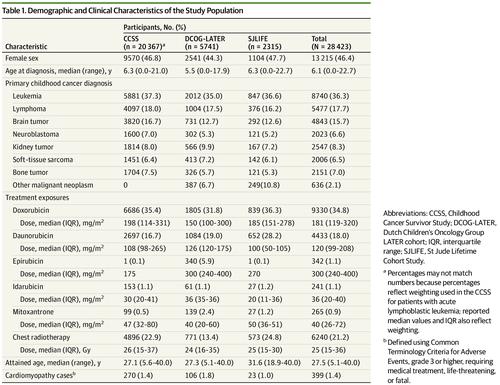
This multicenter cohort study of childhood cancer survivors who survived 5 or more years analyzed data pooled from 20 367 participants in the Childhood Cancer Survivor Study treated from 1970 to 1999, 5741 participants in the Dutch Childhood Oncology Group LATER study diagnosed between 1963 and 2001, and 2315 participants in the St Jude Lifetime study treated from 1962 to 2005.
Exposures
Cumulative doses of each agent (the anthracyclines doxorubicin, daunorubicin, epirubicin, and idarubicin; and the anthraquinone mitoxantrone) along with chest radiotherapy exposure were abstracted from medical records.
Main Outcomes and Measures
Cardiomyopathy (severe, life-threatening, or fatal) by 40 years of age. Agent-specific Cox proportional hazards models evaluated cardiomyopathy risk, adjusting for chest radiotherapy, age at cancer diagnosis, sex, and exposure to anthracyclines or to an anthraquinone. An agent-specific cardiomyopathy equivalence ratio (relative to doxorubicin) was estimated for each dose category as a ratio of the hazard ratios, and then a weighted mean determined the overall agent-specific equivalence ratio across all dose categories.
Results
Of 28 423 survivors (46.4% female; median age at cancer diagnosis 6.1 years [range, 0.0-22.7 years]), 9330 patients received doxorubicin, 4433 received daunorubicin, 342 received epirubicin, 241 received idarubicin, and 265 received mitoxantrone. After a median follow-up of 20.0 years (range, 5.0-40.0 years) following receipt of a cancer diagnosis, 399 cardiomyopathy cases were observed. Relative to doxorubicin, the equivalence ratios were 0.6 (95% CI, 0.4-1.0) for daunorubicin, 0.8 (95% CI, 0.5-2.8) for epirubicin, and 10.5 (95% CI, 6.2-19.1) for mitoxantrone. Outcomes were too rare to generate idarubicin-specific estimates. Ratios based on a continuous linear dose-response relationship were similar for daunorubicin (0.5 [95% CI, 0.4-0.7]) and epirubicin (0.8 [95% CI, 0.3-1.4]). The relationship between mitoxantrone and doxorubicin appeared better characterized by a linear exponential model.
Conclusions and Relevance
In a large data set assembled to examine long-term cardiomyopathy risk in childhood cancer survivors, daunorubicin was associated with decreased cardiomyopathy risk vs doxorubicin, whereas epirubicin was approximately isoequivalent. By contrast, the current hematologic-based doxorubicin dose equivalency of mitoxantrone (4:1) appeared to significantly underestimate the association of mitoxantrone with long-term cardiomyopathy risk.
中文翻译:

推导蒽环类和蒽醌与多柔比星晚发心脏毒性的当量比。
重要性 蒽环类药物是许多有效的儿科癌症治疗方案的一部分。大多数儿科肿瘤治疗小组认为蒽环类药物的血液毒性与其心脏毒性相当;例如,儿童肿瘤学组替代规则认为柔红霉素和表柔比星与多柔比星等效,而米托蒽醌和伊达比星被认为毒性是多柔比星的 4 至 5 倍。目的 确定阿霉素与其他蒽环类药物或蒽醌米托蒽醌治疗迟发性心肌病的最佳剂量当量比。设计、设置和参与者 这项针对存活 5 年或以上的儿童癌症幸存者的多中心队列研究分析了 1970 年至 1999 年间接受治疗的儿童癌症幸存者研究中 20 367 名参与者的数据,荷兰儿童肿瘤学小组后来的研究中诊断出 5741 名参与者1963 年至 2001 年期间的数据,以及 1962 年至 2005 年期间接受治疗的 St Jude Lifetime 研究中的 2315 名参与者。 暴露 提取了每种药物(蒽环类阿霉素、柔红霉素、表柔比星和伊达比星;以及蒽醌米托蒽醌)的累积剂量以及胸部放射治疗暴露从医疗记录中。主要结果和措施 40 岁之前发生心肌病(严重、危及生命或致命)。特定药物的 Cox 比例风险模型评估了心肌病风险,调整了胸部放疗、癌症诊断时的年龄、性别以及蒽环类药物或蒽醌的暴露。 将每个剂量类别的药物特异性心肌病当量比(相对于阿霉素)估计为风险比的比率,然后加权平均值确定所有剂量类别的总体药物特异性当量比。结果 在 28,423 名幸存者中(46.4% 为女性;癌症诊断时的中位年龄为 6.1 岁 [范围,0.0-22.7 岁]),9330 名患者接受了阿霉素治疗,4433 名患者接受了柔红霉素治疗,342 名患者接受了表阿霉素治疗,241 名患者接受了伊达比星治疗,265 名患者接受了米托蒽醌治疗。在收到癌症诊断后中位随访 20.0 年(范围为 5.0-40.0 年)后,观察到 399 例心肌病病例。相对于阿霉素,柔红霉素的当量比为 0.6(95% CI,0.4-1.0),表阿霉素为 0.8(95% CI,0.5-2.8),米托蒽醌为 10.5(95% CI,6.2-19.1)。结果太罕见,无法生成特定于伊达比星的估计值。基于连续线性剂量反应关系的比率对于柔红霉素(0.5 [95% CI,0.4-0.7])和表阿霉素(0.8 [95% CI,0.3-1.4])相似。米托蒽醌和阿霉素之间的关系似乎可以通过线性指数模型更好地表征。结论和相关性 在为检查儿童癌症幸存者的长期心肌病风险而收集的大型数据集中,与阿霉素相比,柔红霉素可降低心肌病风险,而表柔比星则大致相同。相比之下,目前基于血液学的阿霉素与米托蒽醌的剂量当量(4:1)似乎明显低估了米托蒽醌与长期心肌病风险的关联。
更新日期:2019-06-14
中文翻译:

推导蒽环类和蒽醌与多柔比星晚发心脏毒性的当量比。
重要性 蒽环类药物是许多有效的儿科癌症治疗方案的一部分。大多数儿科肿瘤治疗小组认为蒽环类药物的血液毒性与其心脏毒性相当;例如,儿童肿瘤学组替代规则认为柔红霉素和表柔比星与多柔比星等效,而米托蒽醌和伊达比星被认为毒性是多柔比星的 4 至 5 倍。目的 确定阿霉素与其他蒽环类药物或蒽醌米托蒽醌治疗迟发性心肌病的最佳剂量当量比。设计、设置和参与者 这项针对存活 5 年或以上的儿童癌症幸存者的多中心队列研究分析了 1970 年至 1999 年间接受治疗的儿童癌症幸存者研究中 20 367 名参与者的数据,荷兰儿童肿瘤学小组后来的研究中诊断出 5741 名参与者1963 年至 2001 年期间的数据,以及 1962 年至 2005 年期间接受治疗的 St Jude Lifetime 研究中的 2315 名参与者。 暴露 提取了每种药物(蒽环类阿霉素、柔红霉素、表柔比星和伊达比星;以及蒽醌米托蒽醌)的累积剂量以及胸部放射治疗暴露从医疗记录中。主要结果和措施 40 岁之前发生心肌病(严重、危及生命或致命)。特定药物的 Cox 比例风险模型评估了心肌病风险,调整了胸部放疗、癌症诊断时的年龄、性别以及蒽环类药物或蒽醌的暴露。 将每个剂量类别的药物特异性心肌病当量比(相对于阿霉素)估计为风险比的比率,然后加权平均值确定所有剂量类别的总体药物特异性当量比。结果 在 28,423 名幸存者中(46.4% 为女性;癌症诊断时的中位年龄为 6.1 岁 [范围,0.0-22.7 岁]),9330 名患者接受了阿霉素治疗,4433 名患者接受了柔红霉素治疗,342 名患者接受了表阿霉素治疗,241 名患者接受了伊达比星治疗,265 名患者接受了米托蒽醌治疗。在收到癌症诊断后中位随访 20.0 年(范围为 5.0-40.0 年)后,观察到 399 例心肌病病例。相对于阿霉素,柔红霉素的当量比为 0.6(95% CI,0.4-1.0),表阿霉素为 0.8(95% CI,0.5-2.8),米托蒽醌为 10.5(95% CI,6.2-19.1)。结果太罕见,无法生成特定于伊达比星的估计值。基于连续线性剂量反应关系的比率对于柔红霉素(0.5 [95% CI,0.4-0.7])和表阿霉素(0.8 [95% CI,0.3-1.4])相似。米托蒽醌和阿霉素之间的关系似乎可以通过线性指数模型更好地表征。结论和相关性 在为检查儿童癌症幸存者的长期心肌病风险而收集的大型数据集中,与阿霉素相比,柔红霉素可降低心肌病风险,而表柔比星则大致相同。相比之下,目前基于血液学的阿霉素与米托蒽醌的剂量当量(4:1)似乎明显低估了米托蒽醌与长期心肌病风险的关联。

































 京公网安备 11010802027423号
京公网安备 11010802027423号