当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Cation on the Morphology of Boehmite Precipitated from Alkaline Solutions by Adding Gibbsite as Seed
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2019-01-31 00:00:00 , DOI: 10.1021/acs.cgd.8b01756 Zheng Li , Guihua Liu , Xiaobin Li , Tiangui Qi , Zhihong Peng , Qiusheng Zhou
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2019-01-31 00:00:00 , DOI: 10.1021/acs.cgd.8b01756 Zheng Li , Guihua Liu , Xiaobin Li , Tiangui Qi , Zhihong Peng , Qiusheng Zhou
|
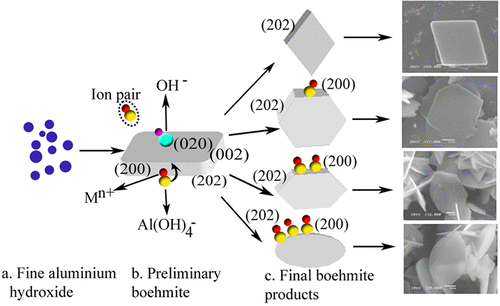
Regulation of boehmite morphology is important for improving its performance in applications. In this paper, the dependence of boehmite morphology on cation ions was studied by XRD, SEM, TEM, FTIR, and zeta-potential, together with calculation of ion degree of association. Boehmite was respectively precipitated from sodium, potassium, lithium, or barium alkaline solution by adding gibbsite as seed at 180 °C for 2 h. Cation concentration and species determined the boehmite morphology. Increase in cation concentration or precipitation in K+, Na+, Li+, and Ba2+ alkaline solution correspondingly precipitated rhombic, hexagonal, and elliptical morphology for boehmite. Al(OH)4– was predominantly found in filtrate solution by Raman spectra, whereas red shift of FTIR was observed in alkaline solution containing Ba2+, Li+, Na+, and K+ cations. The degree of association of Mn+[Al(OH)4–]nn− increased in the following order: Ba2+[Al(OH)4–]2 > Li+Al(OH)4– > K+Al(OH)4– > Na+Al(OH)4– in the same concentration of cation solution. This finding agreed with the variations in FTIR change and zeta potential. Meanwhile, increasing cation and Al(OH)4– concentration or precipitation in lithium or barium alkaline solution caused the (202) facet of boehmite to gradually disappear and the (200) facet to be exposed. Therefore, the selective adsorption of ion pairs on the (202) and (200) facet of boehmite mainly accounted for the boehmite morphology.
中文翻译:

阳离子对菱镁矿添加碱溶液沉淀的勃姆石形态的影响
勃姆石形态的调节对于提高其在应用中的性能很重要。本文通过XRD,SEM,TEM,FTIR和zeta势研究了勃姆石形态对阳离子的依赖性,并计算了离子的缔合度。通过在180°C下添加三水铝石作为晶种2 h,分别从钠,钾,锂或钡碱性溶液中沉淀勃姆石。阳离子浓度和物种决定了勃姆石的形态。阳离子浓度的增加或在K +,Na +,Li +和Ba 2+碱性溶液中的沉淀相应地沉淀了勃姆石的菱形,六角形和椭圆形形态。Al(OH)4 –在拉曼光谱中主要在滤液中发现FTIR,而在含有Ba 2 +,Li +,Na +和K +阳离子的碱性溶液中观察到FTIR的红移。M的关联度Ñ + [铝(OH)4 - ] ñ ñ -巴:增加以下顺序2+ [铝(OH)4 - ] 2 >锂+的Al(OH)4 - >ķ +铝(OH)4 – > Na + Al(OH)4 –在相同浓度的阳离子溶液中。这一发现与FTIR变化和ζ电位的变化一致。同时,增加阳离子和Al(OH)4 -锂或钡的碱性溶液的浓度或沉淀引起勃姆石的(202)小面逐渐消失和待曝光的(200)面。因此,离子对在勃姆石的(202)和(200)面上的选择性吸附主要是勃姆石的形态。
更新日期:2019-01-31
中文翻译:

阳离子对菱镁矿添加碱溶液沉淀的勃姆石形态的影响
勃姆石形态的调节对于提高其在应用中的性能很重要。本文通过XRD,SEM,TEM,FTIR和zeta势研究了勃姆石形态对阳离子的依赖性,并计算了离子的缔合度。通过在180°C下添加三水铝石作为晶种2 h,分别从钠,钾,锂或钡碱性溶液中沉淀勃姆石。阳离子浓度和物种决定了勃姆石的形态。阳离子浓度的增加或在K +,Na +,Li +和Ba 2+碱性溶液中的沉淀相应地沉淀了勃姆石的菱形,六角形和椭圆形形态。Al(OH)4 –在拉曼光谱中主要在滤液中发现FTIR,而在含有Ba 2 +,Li +,Na +和K +阳离子的碱性溶液中观察到FTIR的红移。M的关联度Ñ + [铝(OH)4 - ] ñ ñ -巴:增加以下顺序2+ [铝(OH)4 - ] 2 >锂+的Al(OH)4 - >ķ +铝(OH)4 – > Na + Al(OH)4 –在相同浓度的阳离子溶液中。这一发现与FTIR变化和ζ电位的变化一致。同时,增加阳离子和Al(OH)4 -锂或钡的碱性溶液的浓度或沉淀引起勃姆石的(202)小面逐渐消失和待曝光的(200)面。因此,离子对在勃姆石的(202)和(200)面上的选择性吸附主要是勃姆石的形态。































 京公网安备 11010802027423号
京公网安备 11010802027423号