当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Understanding the Roles of Dissolution and Diffusion in Cr(OH)3 Oxidation by δ-MnO2
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-01-30 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00129 Chao Pan 1, 2 , Huan Liu 3 , Jeffrey G. Catalano 4 , Zimeng Wang 5 , Ao Qian 6 , Daniel E. Giammar 2
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-01-30 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00129 Chao Pan 1, 2 , Huan Liu 3 , Jeffrey G. Catalano 4 , Zimeng Wang 5 , Ao Qian 6 , Daniel E. Giammar 2
Affiliation
|
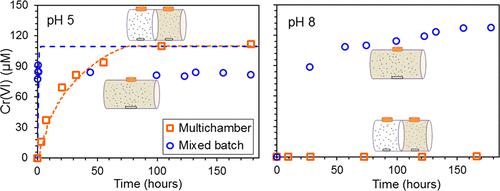
Manganese oxides are the major oxidants of Cr(III) to Cr(VI) in natural environments. This study evaluated the rate and extent of oxidation of Cr(III) released from Cr(OH)3 by δ-MnO2 from pH 5 to 9 with a particular focus on quantifying the rate constant for Cr(III) oxidation on the MnO2 surface. The Cr(III) oxidation rate was initially fast, but it then slowed and ceased for pH 5 to pH 7, which agrees with previously reported inhibition of the redox reaction above pH 4 by precipitation of Cr(III) on the MnO2 surface. Above pH 7, overall Cr(VI) production was higher than at lower pH even though the dissolved Cr(III) concentration in equilibrium with Cr(OH)3 was lower. This is probably due to the reoxidation of aqueous Mn(II) by dissolved oxygen at higher pH, which made more manganese oxide available to oxidize Cr(III) to Cr(VI). Multichamber experiments were used to assess the role of solid–solid proximity in Cr(OH)3–MnO2 interactions at pH 5. The different rates and extents of Cr(VI) production in the multichamber reactor and completely mixed batch reactor indicate that mixing of Cr(OH)3 and MnO2 solids plays a role in the rate and extent of Cr(VI) generation. Further Cr(VI) generation in this systems strongly depends on pH. Because of the importance of mass transfer of Cr(III) to MnO2 solids for the overall Cr(III) oxidation process, the net rate of Cr(VI) generation in natural environments with Cr(III)-containing solids and MnO2 can be orders of magnitude slower than observed in well-mixed laboratory-scale batch experiments.
中文翻译:

了解溶解和扩散的以Cr(OH)中的作用3由氧化δ-的MnO 2
锰氧化物是自然环境中Cr(III)到Cr(VI)的主要氧化剂。本研究评估选自Cr(OH)释放的速率和Cr(III)氧化的程度3由δ-的MnO 2从pH5至9与特定焦点上量化为铬(III)的速率常数氧化上的MnO 2表面。Cr(III)的氧化速率最初是很快的,但随后放慢了速度,并在pH 5到pH 7时停止了,这与先前报道的通过在MnO 2表面上沉积Cr(III)抑制pH 4以上的氧化还原反应相吻合。pH高于7时,即使溶解的Cr(III)浓度与Cr(OH)3处于平衡状态,总的Cr(VI)产量仍高于较低pH值。较低。这可能是由于Mn(II)水溶液在较高的pH值下被溶解的氧再氧化所致,这使得更多的锰氧化物可用于将Cr(III)氧化为Cr(VI)。使用多室实验评估了pH值为5时固-固接近度在Cr(OH)3 -MnO 2相互作用中的作用。多室反应器和完全混合间歇反应器中Cr(VI)产生的速率和程度不同表明混合Cr(OH)3和MnO 2固体的含量在Cr(VI)生成的速率和程度中起作用。该系统中进一步的Cr(VI)生成在很大程度上取决于pH。由于Cr(III)向MnO 2传质的重要性固体用于整个Cr(III)氧化过程,在自然环境中使用含Cr(III)的固体和MnO 2生成Cr(VI)的净速率可能比在实验室中充分混合的批次中观察到的速度慢几个数量级实验。
更新日期:2019-01-30
中文翻译:

了解溶解和扩散的以Cr(OH)中的作用3由氧化δ-的MnO 2
锰氧化物是自然环境中Cr(III)到Cr(VI)的主要氧化剂。本研究评估选自Cr(OH)释放的速率和Cr(III)氧化的程度3由δ-的MnO 2从pH5至9与特定焦点上量化为铬(III)的速率常数氧化上的MnO 2表面。Cr(III)的氧化速率最初是很快的,但随后放慢了速度,并在pH 5到pH 7时停止了,这与先前报道的通过在MnO 2表面上沉积Cr(III)抑制pH 4以上的氧化还原反应相吻合。pH高于7时,即使溶解的Cr(III)浓度与Cr(OH)3处于平衡状态,总的Cr(VI)产量仍高于较低pH值。较低。这可能是由于Mn(II)水溶液在较高的pH值下被溶解的氧再氧化所致,这使得更多的锰氧化物可用于将Cr(III)氧化为Cr(VI)。使用多室实验评估了pH值为5时固-固接近度在Cr(OH)3 -MnO 2相互作用中的作用。多室反应器和完全混合间歇反应器中Cr(VI)产生的速率和程度不同表明混合Cr(OH)3和MnO 2固体的含量在Cr(VI)生成的速率和程度中起作用。该系统中进一步的Cr(VI)生成在很大程度上取决于pH。由于Cr(III)向MnO 2传质的重要性固体用于整个Cr(III)氧化过程,在自然环境中使用含Cr(III)的固体和MnO 2生成Cr(VI)的净速率可能比在实验室中充分混合的批次中观察到的速度慢几个数量级实验。











































 京公网安备 11010802027423号
京公网安备 11010802027423号