当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu-Based Single-Atom Catalysts Boost Electroreduction of CO2 to CH3OH: First-Principles Predictions
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-02-08 , DOI: 10.1021/acs.jpcc.8b12449 Zhonglong Zhao 1 , Gang Lu 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-02-08 , DOI: 10.1021/acs.jpcc.8b12449 Zhonglong Zhao 1 , Gang Lu 1
Affiliation
|
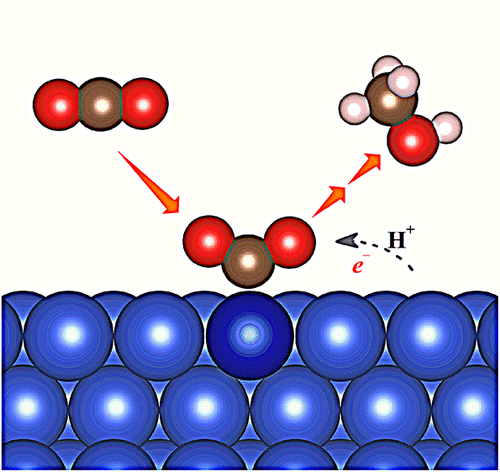
Electrochemical CO2 reduction reaction (CO2RR) to fuels represents one of the most attractive approaches to mitigate our pressing energy and environmental threats. Cu is the best known metal catalyst that can produce an appreciable amount of hydrocarbons from CO2, but it suffers from a high overpotential and poor selectivity. In this work, by means of first-principles calculations, we predict that Cu-based single-atom alloys (SAAs) could be exceptional electrocatalysts for CO2RR. In particular, we predict that Co@Cu SAA could be a promising catalyst on which methanol can be produced at a low overpotential and high selectivity. The isolated Co atoms lead to a narrowed d-band and an upshifted d-band center which can stabilize chemisorbed CO2 on a surface, significantly lowering the reaction barrier. The narrowed Co d-band increases the bonding to a key intermediate, which in turn eliminates the need for its migration and enables a selective and efficient production of CH3OH through the pathway of CO2 → COOH* → CO* → COH* → CHOH* → CH2OH* → CH3OH.
中文翻译:

铜基单原子催化剂促进CO 2还原为CH 3 OH的电还原:第一性原理预测
燃料的电化学CO 2还原反应(CO 2 RR)代表了减轻我们紧迫的能源和环境威胁的最有吸引力的方法之一。Cu是最公知的金属催化剂,其可以从CO 2产生相当数量的烃,但是它具有高的过电势和较差的选择性。在这项工作中,通过第一性原理的计算,我们预测铜基单原子合金(SAA)可能是CO 2的特殊电催化剂。RR。特别地,我们预测Co @ Cu SAA可能是一种有前途的催化剂,在该催化剂上可以低超电势和高选择性生产甲醇。孤立的Co原子导致d谱带变窄和d谱带中心向上偏移,这可以使化学吸附的CO 2在表面上稳定,从而显着降低反应势垒。狭窄的Co d带增加了与关键中间体的键合,从而消除了对其迁移的需要,并能够通过CO 2 →COOH *→CO *→COH *→的途径选择性而高效地生产CH 3 OH CHOH *→CH 2 OH *→CH 3 OH。
更新日期:2019-02-08
中文翻译:

铜基单原子催化剂促进CO 2还原为CH 3 OH的电还原:第一性原理预测
燃料的电化学CO 2还原反应(CO 2 RR)代表了减轻我们紧迫的能源和环境威胁的最有吸引力的方法之一。Cu是最公知的金属催化剂,其可以从CO 2产生相当数量的烃,但是它具有高的过电势和较差的选择性。在这项工作中,通过第一性原理的计算,我们预测铜基单原子合金(SAA)可能是CO 2的特殊电催化剂。RR。特别地,我们预测Co @ Cu SAA可能是一种有前途的催化剂,在该催化剂上可以低超电势和高选择性生产甲醇。孤立的Co原子导致d谱带变窄和d谱带中心向上偏移,这可以使化学吸附的CO 2在表面上稳定,从而显着降低反应势垒。狭窄的Co d带增加了与关键中间体的键合,从而消除了对其迁移的需要,并能够通过CO 2 →COOH *→CO *→COH *→的途径选择性而高效地生产CH 3 OH CHOH *→CH 2 OH *→CH 3 OH。































 京公网安备 11010802027423号
京公网安备 11010802027423号