当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glycopeptide Synthesis Based on a TFA‐Labile Protection Strategy and One‐Pot Four‐Segment Ligation for the Synthesis of O‐Glycosylated Histone H2A
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2019-02-19 , DOI: 10.1002/ejoc.201801885 Yuya Asahina 1 , Toru Kawakami 1 , Hironobu Hojo 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2019-02-19 , DOI: 10.1002/ejoc.201801885 Yuya Asahina 1 , Toru Kawakami 1 , Hironobu Hojo 1
Affiliation
|
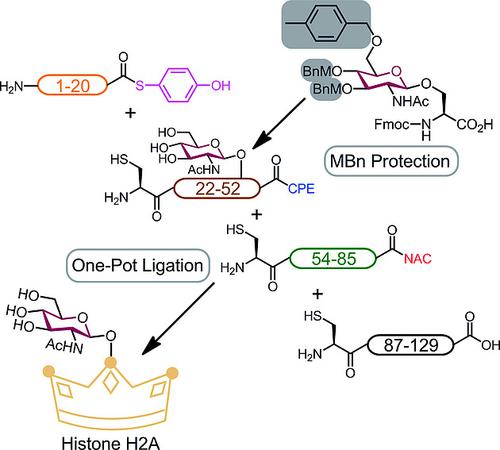
O‐glycosylated protein–histone H2A– was chemically synthesized by novel two methodologies: 1) the TFA‐labile protection strategy using MBn group for a facile glycopeptide synthesis; 2) the one‐pot four‐peptide‐segment ligation using the peptide thioester and two orthogonal thioester equivalents. These methods provided more efficient synthesis to obtain H2A having O‐GlcNAc at Ser.[40]

中文翻译:

基于TFA不稳定保护策略和一罐四段连接的糖肽合成,用于合成O-糖基化组蛋白H2A
O-糖基化蛋白-组蛋白H2A-是通过两种新的化学方法化学合成的:1)使用MBn基团进行TFA不稳定的保护策略,以实现简便的糖肽合成;2)使用肽硫酯和两个正交硫酯等效物进行一锅四肽段连接。这些方法提供了更有效的合成方法,以获得在Ser上具有O- GlcNAc的H2A 。[40]

更新日期:2019-02-19

中文翻译:

基于TFA不稳定保护策略和一罐四段连接的糖肽合成,用于合成O-糖基化组蛋白H2A
O-糖基化蛋白-组蛋白H2A-是通过两种新的化学方法化学合成的:1)使用MBn基团进行TFA不稳定的保护策略,以实现简便的糖肽合成;2)使用肽硫酯和两个正交硫酯等效物进行一锅四肽段连接。这些方法提供了更有效的合成方法,以获得在Ser上具有O- GlcNAc的H2A 。[40]
































 京公网安备 11010802027423号
京公网安备 11010802027423号