当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Ynamide Additions to Isatins: Concise Access to Oxindole Alkaloids.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-02-14 , DOI: 10.1002/anie.201814074
Max Moskowitz 1 , Christian Wolf 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-02-14 , DOI: 10.1002/anie.201814074
Max Moskowitz 1 , Christian Wolf 1
Affiliation
|
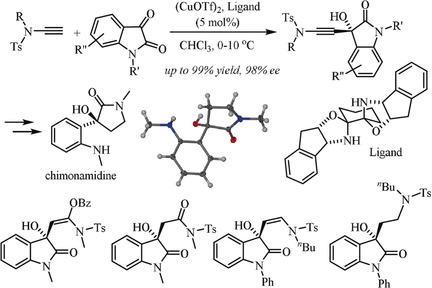
The highly enantioselective addition of terminal ynamides to a variety of isatins, catalyzed by a bisoxazolidine copper complex under mild, base‐free reaction conditions, is described. The reaction is broad in scope, scalable, applicable to unprotected isatins, and provides efficient access to 3‐hydroxyoxindoles carrying a tetrasubstituted chiral center with excellent yields and enantioselectivities. Synthetically versatile, multifunctional 3‐hydroxyindolinones are obtained by hydration, partial hydrogenation, or hydroxyacyloxylation of the ynamide moiety at room temperature and exhaustive hydrogenation followed by reductive detosylation and spontaneous cyclization affords cinchonamidine alkaloids.
中文翻译:

靛红的催化对映选择性 Ynamide 加成:简要获取 Oxindole 生物碱。
描述了在温和的无碱反应条件下,由双恶唑烷铜配合物催化,末端炔酰胺高度对映选择性地加成到各种靛红上。该反应范围广泛,可扩展,适用于未受保护的靛红,并以优异的产率和对映选择性有效地获得带有四取代手性中心的3-羟基吲哚。合成通用的多功能 3-羟基二氢吲哚酮是通过在室温下对 ynamide 部分进行水合、部分氢化或羟基酰氧基化以及彻底氢化,然后进行还原去甲苯基化和自发环化来获得辛可脒生物碱。
更新日期:2019-02-14
中文翻译:

靛红的催化对映选择性 Ynamide 加成:简要获取 Oxindole 生物碱。
描述了在温和的无碱反应条件下,由双恶唑烷铜配合物催化,末端炔酰胺高度对映选择性地加成到各种靛红上。该反应范围广泛,可扩展,适用于未受保护的靛红,并以优异的产率和对映选择性有效地获得带有四取代手性中心的3-羟基吲哚。合成通用的多功能 3-羟基二氢吲哚酮是通过在室温下对 ynamide 部分进行水合、部分氢化或羟基酰氧基化以及彻底氢化,然后进行还原去甲苯基化和自发环化来获得辛可脒生物碱。































 京公网安备 11010802027423号
京公网安备 11010802027423号