当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Conversion of CO2 to 2-Bromoethanol in a Membraneless Cell
ACS Energy Letters ( IF 19.3 ) Pub Date : 2019-01-29 00:00:00 , DOI: 10.1021/acsenergylett.9b00004 Shenghong Zhong 1 , Zhen Cao 1 , Xiulin Yang 1 , Sergey M. Kozlov 1 , Kuo-Wei Huang 1 , Vincent Tung 1 , Luigi Cavallo 1 , Lain-Jong Li 1 , Yu Han 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2019-01-29 00:00:00 , DOI: 10.1021/acsenergylett.9b00004 Shenghong Zhong 1 , Zhen Cao 1 , Xiulin Yang 1 , Sergey M. Kozlov 1 , Kuo-Wei Huang 1 , Vincent Tung 1 , Luigi Cavallo 1 , Lain-Jong Li 1 , Yu Han 1
Affiliation
|
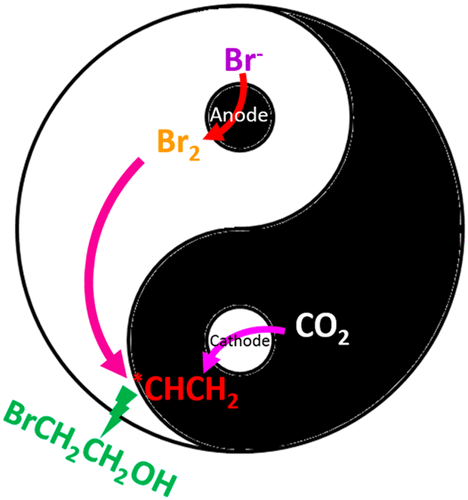
Tremendous research efforts have been made to electrochemically convert CO2 into useful chemicals, with the main products being limited to hydrocarbons and oxygenates. One-step processes that integrate CO2 reduction with a subsequent reaction to produce other types of functional chemicals are economically attractive. Here we report that direct electrochemical conversion of CO2 to 2-bromoethanol, a valuable pharmaceutical intermediate, is enabled by coupling the anodic and cathodic reactions with the presence of potassium bromide electrolyte in a membraneless electrochemical cell. The maximum Faradaic efficiency of converting CO2 to 2-bromoethanol that we achieved is 40% at −1.01 VRHE with a partial current density of −19 mA cm–2. Our work demonstrates a new strategy for making value-added products from CO2 through a simple process.
中文翻译:

无膜电解槽中CO 2到2-溴乙醇的电化学转化
为了将CO 2电化学转化为有用的化学物质,已经进行了大量的研究工作,其主要产物仅限于碳氢化合物和含氧化合物。将CO 2还原与随后的反应结合起来以生产其他类型的功能化学品的一步法在经济上具有吸引力。在这里,我们报告说,通过在无膜电化学电池中将阳极和阴极反应与溴化钾电解质的存在耦合,可以将CO 2直接电化学转化为有价值的药物中间体2-溴乙醇。在−1.01 V RHE时,我们实现的将CO 2转化为2-溴乙醇的最大法拉第效率为40%部分电流密度为−19 mA cm –2。我们的工作展示了一种通过简单过程从CO 2制造增值产品的新策略。
更新日期:2019-01-29
中文翻译:

无膜电解槽中CO 2到2-溴乙醇的电化学转化
为了将CO 2电化学转化为有用的化学物质,已经进行了大量的研究工作,其主要产物仅限于碳氢化合物和含氧化合物。将CO 2还原与随后的反应结合起来以生产其他类型的功能化学品的一步法在经济上具有吸引力。在这里,我们报告说,通过在无膜电化学电池中将阳极和阴极反应与溴化钾电解质的存在耦合,可以将CO 2直接电化学转化为有价值的药物中间体2-溴乙醇。在−1.01 V RHE时,我们实现的将CO 2转化为2-溴乙醇的最大法拉第效率为40%部分电流密度为−19 mA cm –2。我们的工作展示了一种通过简单过程从CO 2制造增值产品的新策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号