当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Insights into the Catalytic Mechanism of Bacterial Carboxylic Acid Reductase.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2019-02-12 , DOI: 10.1021/acs.jcim.8b00763
Ge Qu 1 , Mingxing Fu 2 , Lili Zhao 2 , Beibei Liu 1 , Pi Liu 1 , Wenchao Fan 1 , Jun-An Ma 3 , Zhoutong Sun 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2019-02-12 , DOI: 10.1021/acs.jcim.8b00763
Ge Qu 1 , Mingxing Fu 2 , Lili Zhao 2 , Beibei Liu 1 , Pi Liu 1 , Wenchao Fan 1 , Jun-An Ma 3 , Zhoutong Sun 1
Affiliation
|
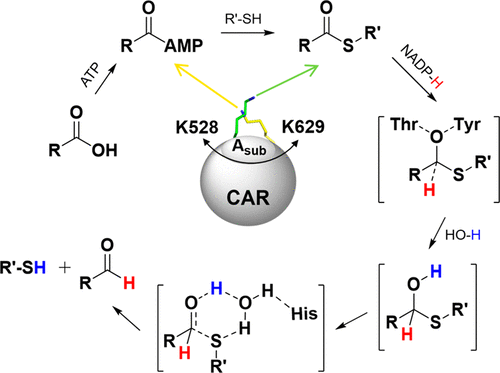
Multidomain carboxylic acid reductases (CARs) can reduce a wide range of carboxylic acids to the corresponding aldehydes in the presence of ATP and NADPH. Recent X-ray structures of the individual (di)domains of Segniliparus rugosus CAR (SrCAR) shed light on the catalysis mechanism and revealed domain dynamics during the different states of the reaction. However, the details of the catalytic mechanism of each step operated by the corresponding domains are still elusive. Toward this end, several models based on the crystal structures were constructed, and molecular dynamics simulations along with density functional theory (DFT) calculations were employed to elucidate the conformational dynamics and catalytic mechanism of SrCAR concealed to static crystallography. We investigated the roles of the key residues in the substrate binding pocket involved in the adenylation and thiolation reactions and especially determined the roles played by a nonconserved Lys528 residue in the thiolation step, which were further verified by site-directed mutagenesis. The reduction mechanism of SrCAR, including the natures of the transition states for hydride and proton transfer, was also explored theoretically using the DFT method B3LYP. The information presented here is useful as a guide for the future rational design of CARs.
中文翻译:

细菌羧酸还原酶催化机理的计算见解。
在ATP和NADPH的存在下,多域羧酸还原酶(CARs)可以将多种羧酸还原为相应的醛。皱叶小球藻CAR(SrCAR)的单个(二)域的最新X射线结构阐明了催化机理,并揭示了反应不同状态下的域动力学。但是,由相应域操作的每个步骤的催化机理的细节仍然难以捉摸。为此,建立了几种基于晶体结构的模型,并利用分子动力学模拟和密度泛函理论(DFT)计算来阐明隐含在静态晶体学中的SrCAR的构象动力学和催化机理。我们调查了参与腺苷酸化和硫醇化反应的底物结合口袋中关键残基的作用,并特别确定了非保守的Lys528残基在硫醇化步骤中所起的作用,这已通过定点诱变得到进一步证实。还使用DFT方法B3LYP从理论上探讨了SrCAR的还原机理,包括氢化物和质子转移的过渡态的性质。此处提供的信息对于将来CAR的合理设计非常有用。还使用DFT方法B3LYP在理论上探索了包括氢化物和质子转移的过渡态性质在内的所有元素。此处提供的信息对于将来CAR的合理设计非常有用。还使用DFT方法B3LYP在理论上探索了包括氢化物和质子转移的过渡态性质在内的所有元素。此处提供的信息对于将来CAR的合理设计非常有用。
更新日期:2019-01-28
中文翻译:

细菌羧酸还原酶催化机理的计算见解。
在ATP和NADPH的存在下,多域羧酸还原酶(CARs)可以将多种羧酸还原为相应的醛。皱叶小球藻CAR(SrCAR)的单个(二)域的最新X射线结构阐明了催化机理,并揭示了反应不同状态下的域动力学。但是,由相应域操作的每个步骤的催化机理的细节仍然难以捉摸。为此,建立了几种基于晶体结构的模型,并利用分子动力学模拟和密度泛函理论(DFT)计算来阐明隐含在静态晶体学中的SrCAR的构象动力学和催化机理。我们调查了参与腺苷酸化和硫醇化反应的底物结合口袋中关键残基的作用,并特别确定了非保守的Lys528残基在硫醇化步骤中所起的作用,这已通过定点诱变得到进一步证实。还使用DFT方法B3LYP从理论上探讨了SrCAR的还原机理,包括氢化物和质子转移的过渡态的性质。此处提供的信息对于将来CAR的合理设计非常有用。还使用DFT方法B3LYP在理论上探索了包括氢化物和质子转移的过渡态性质在内的所有元素。此处提供的信息对于将来CAR的合理设计非常有用。还使用DFT方法B3LYP在理论上探索了包括氢化物和质子转移的过渡态性质在内的所有元素。此处提供的信息对于将来CAR的合理设计非常有用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号