Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An All‐Solid‐State Rechargeable Chloride Ion Battery
Advanced Science ( IF 14.3 ) Pub Date : 2019-01-28 , DOI: 10.1002/advs.201802130 Chao Chen 1 , Tingting Yu 1 , Meng Yang 1 , Xiangyu Zhao 1 , Xiaodong Shen 1, 2
Advanced Science ( IF 14.3 ) Pub Date : 2019-01-28 , DOI: 10.1002/advs.201802130 Chao Chen 1 , Tingting Yu 1 , Meng Yang 1 , Xiangyu Zhao 1 , Xiaodong Shen 1, 2
Affiliation
|
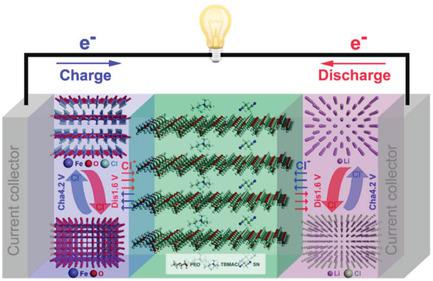
The chloride ion battery has been developed as one of the alternative battery chemistries beyond lithium ion, toward abundant material resources and high energy density. Its application, however, is limited by the dissolution of electrode materials and side reactions in the liquid electrolyte. Herein, a solid polymer electrolyte allowing chloride ion transfer and consisting of poly(ethylene oxide) as the polymer matrix, tributylmethylammonium chloride as the chloride salt, and succinonitrile as the solid plasticizer is reported. The as‐prepared polymer electrolyte shows conductivities of 10−5–10−4 S cm−1 in the temperature range of 298–343 K. When it is assembled with the iron oxychloride/lithium electrode system, reversible electrochemical redox reactions of FeOCl/FeO at the cathode side and Li/LiCl at the anode side are realized, demonstrating the first all‐solid‐state rechargeable chloride ion battery.
中文翻译:

全固态可充电氯离子电池
氯离子电池已被开发为锂离子之外的替代化学电池之一,朝着丰富的材料资源和高能量密度的方向发展。然而,其应用受到电极材料的溶解和液体电解质中的副反应的限制。本文报道了一种允许氯离子转移的固体聚合物电解质,其由作为聚合物基质的聚环氧乙烷、作为氯化物盐的三丁基甲基氯化铵和作为固体增塑剂的丁二腈组成。所制备的聚合物电解质在298-343 K的温度范围内表现出10 -5 –10 -4 S cm -1的电导率。当它与铁氧氯化物/锂电极系统组装时,发生FeOCl/的可逆电化学氧化还原反应。实现了阴极侧的 FeO 和阳极侧的 Li/LiCl,展示了第一个全固态可充电氯离子电池。
更新日期:2019-01-28
中文翻译:

全固态可充电氯离子电池
氯离子电池已被开发为锂离子之外的替代化学电池之一,朝着丰富的材料资源和高能量密度的方向发展。然而,其应用受到电极材料的溶解和液体电解质中的副反应的限制。本文报道了一种允许氯离子转移的固体聚合物电解质,其由作为聚合物基质的聚环氧乙烷、作为氯化物盐的三丁基甲基氯化铵和作为固体增塑剂的丁二腈组成。所制备的聚合物电解质在298-343 K的温度范围内表现出10 -5 –10 -4 S cm -1的电导率。当它与铁氧氯化物/锂电极系统组装时,发生FeOCl/的可逆电化学氧化还原反应。实现了阴极侧的 FeO 和阳极侧的 Li/LiCl,展示了第一个全固态可充电氯离子电池。































 京公网安备 11010802027423号
京公网安备 11010802027423号