当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Limited Role of Electronic Support Effects in Selective Alkyne Hydrogenation: A Kinetic Study of Au/MOx Catalysts Prepared from Oleylamine‐Capped Colloidal Nanoparticles
ChemCatChem ( IF 3.8 ) Pub Date : 2019-02-28 , DOI: 10.1002/cctc.201801882
James E. Bruno 1 , K. B. Sravan Kumar 2 , Nicolas S. Dwarica 1 , Alexander Hüther 1 , Zhifeng Chen 3 , Clemente S. Guzman 1 , Emily R. Hand 1 , William C. Moore 1 , Robert M. Rioux 3, 4 , Lars C. Grabow 2 , Bert D. Chandler 1
ChemCatChem ( IF 3.8 ) Pub Date : 2019-02-28 , DOI: 10.1002/cctc.201801882
James E. Bruno 1 , K. B. Sravan Kumar 2 , Nicolas S. Dwarica 1 , Alexander Hüther 1 , Zhifeng Chen 3 , Clemente S. Guzman 1 , Emily R. Hand 1 , William C. Moore 1 , Robert M. Rioux 3, 4 , Lars C. Grabow 2 , Bert D. Chandler 1
Affiliation
|
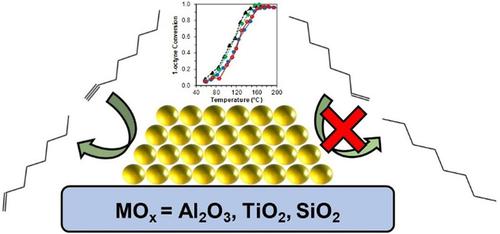
We report a quantitative kinetic evaluation and study of support effects for partial alkyne hydrogenation using oleylamine‐capped Au colloids as catalyst precursors. The amine capping agents can be removed under reducing conditions, generating supported Au nanoparticles of ∼2.5 nm in diameter. The catalysts showed high alkene selectivity (>90 %) at all conversions during alkyne partial hydrogenation. Catalytic activity, observed rate constants, and apparent activation energies (25–40 kJ/mol) were similar for all Au catalysts, indicating support effects are relatively small. Alkyne adsorption, probed with FTIR and DFT, showed adsorption on the support was associated with hydrogen‐bonding interactions. DFT calculations indicate strong alkyne adsorption on Au sites, with the strongest adsorption sites at the metal‐support interface (MSI). The catalysts had similar hydrogen reaction orders (0.7–0.9), and 1‐octyne reaction orders (∼−0.2), suggesting a common mechanism. The reaction kinetics are most consistent with a mechanism involving the non‐competitive activated adsorption of H2 on an alkyne‐covered Au surface.
中文翻译:

电子支持作用在选择性炔烃加氢中的有限作用:由油酸胺封端的胶体纳米颗粒制备的Au / MOx催化剂的动力学研究
我们报告了定量动力学评估,并使用油胺封端的金胶体作为催化剂前体,对部分炔烃加氢的支持作用进行了研究。可以在还原条件下除去胺封端剂,生成直径约2.5 nm的负载型Au纳米颗粒。在炔烃部分加氢期间的所有转化率下,催化剂均显示出较高的烯烃选择性(> 90%)。所有Au催化剂的催化活性,观察到的速率常数和表观活化能(25-40 kJ / mol)均相似,表明载体效应相对较小。用FTIR和DFT探测的炔烃吸附表明,在载体上的吸附与氢键相互作用有关。DFT计算表明,炔在Au位上的吸附力强,在金属-载体界面(MSI)上的吸附力最强。催化剂具有相似的氢反应顺序(0.7-0.9)和1-辛炔反应顺序(〜-0.2),表明其机理相同。反应动力学与涉及非竞争性活化氢吸附的机理最一致2在炔烃覆盖的Au表面上。
更新日期:2019-02-28
中文翻译:

电子支持作用在选择性炔烃加氢中的有限作用:由油酸胺封端的胶体纳米颗粒制备的Au / MOx催化剂的动力学研究
我们报告了定量动力学评估,并使用油胺封端的金胶体作为催化剂前体,对部分炔烃加氢的支持作用进行了研究。可以在还原条件下除去胺封端剂,生成直径约2.5 nm的负载型Au纳米颗粒。在炔烃部分加氢期间的所有转化率下,催化剂均显示出较高的烯烃选择性(> 90%)。所有Au催化剂的催化活性,观察到的速率常数和表观活化能(25-40 kJ / mol)均相似,表明载体效应相对较小。用FTIR和DFT探测的炔烃吸附表明,在载体上的吸附与氢键相互作用有关。DFT计算表明,炔在Au位上的吸附力强,在金属-载体界面(MSI)上的吸附力最强。催化剂具有相似的氢反应顺序(0.7-0.9)和1-辛炔反应顺序(〜-0.2),表明其机理相同。反应动力学与涉及非竞争性活化氢吸附的机理最一致2在炔烃覆盖的Au表面上。































 京公网安备 11010802027423号
京公网安备 11010802027423号