Nature Chemical Biology ( IF 12.9 ) Pub Date : 2019-01-28 , DOI: 10.1038/s41589-018-0222-1 Jean Quancard , Theo Klein , Shan-Yu Fung , Martin Renatus , Nicola Hughes , Laura Israël , John J. Priatel , Sohyeong Kang , Michael A. Blank , Rosa I. Viner , Jutta Blank , Achim Schlapbach , Paul Erbel , Jayachandran Kizhakkedathu , Frédéric Villard , René Hersperger , Stuart E. Turvey , Joerg Eder , Frédéric Bornancin , Christopher M. Overall
|
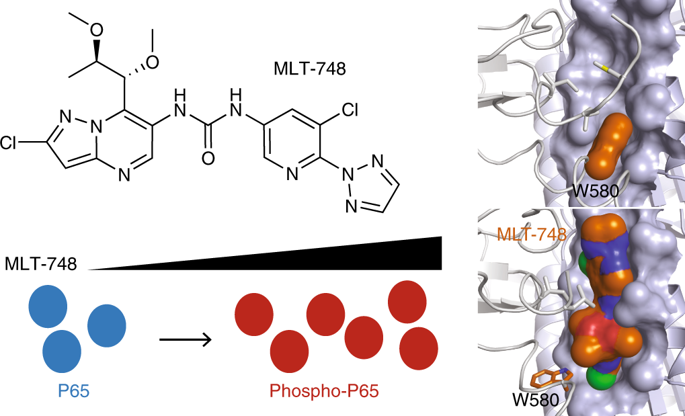
MALT1 paracaspase is central for lymphocyte antigen-dependent responses including NF-κB activation. We discovered nanomolar, selective allosteric inhibitors of MALT1 that bind by displacing the side chain of Trp580, locking the protease in an inactive conformation. Interestingly, we had previously identified a patient homozygous for a MALT1 Trp580-to-serine mutation who suffered from combined immunodeficiency. We show that the loss of tryptophan weakened interactions between the paracaspase and C-terminal immunoglobulin MALT1 domains resulting in protein instability, reduced protein levels and functions. Upon binding of allosteric inhibitors of increasing potency, we found proportionate increased stabilization of MALT1-W580S to reach that of wild-type MALT1. With restored levels of stable MALT1 protein, the most potent of the allosteric inhibitors rescued NF-κB and JNK signaling in patient lymphocytes. Following compound washout, MALT1 substrate cleavage was partly recovered. Thus, a molecular corrector rescues an enzyme deficiency by substituting for the mutated residue, inspiring new potential precision therapies to increase mutant enzyme activity in other deficiencies.
中文翻译:

变构MALT1抑制剂是免疫缺陷患者的分子纠正剂抢救功能
MALT1半胱天冬酶对于淋巴细胞抗原依赖性反应(包括NF-κB激活)至关重要。我们发现MALT1的纳摩尔选择性选择性变构抑制剂可以通过置换Trp580的侧链来结合,从而将蛋白酶锁定在非活性构象中。有趣的是,我们先前已经确定了一名患有MALT1 Trp580到丝氨酸突变纯合的患者,该患者患有合并的免疫缺陷。我们表明色氨酸的损失削弱了半胱天冬酶和C末端免疫球蛋白MALT1域之间的相互作用,从而导致蛋白质不稳定,蛋白质水平和功能降低。通过结合效力增强的变构抑制剂,我们发现MALT1-W580S的稳定度成比例地增加,达到了野生型MALT1的稳定度。恢复稳定的MALT1蛋白水平后,最有效的变构抑制剂可以挽救患者淋巴细胞中的NF-κB和JNK信号。洗脱化合物后,部分回收了MALT1底物的裂解。因此,分子校正剂通过替代突变残基来挽救酶缺乏症,激发出新的潜在精密疗法以增加其他缺陷中的突变酶活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号