当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acid‐Controlled Chemodivergent Synthesis of Three Differently Substituted Quinolines via Site Selective Coupling of ortho‐ Aminoaryl Ketones with α‐Enolic Dithioesters
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-03-31 , DOI: 10.1002/adsc.201500962 Suvajit Koley , Tanmoy Chanda , B. Janaki Ramulu , Sushobhan Chowdhury , Maya Shankar Singh
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-03-31 , DOI: 10.1002/adsc.201500962 Suvajit Koley , Tanmoy Chanda , B. Janaki Ramulu , Sushobhan Chowdhury , Maya Shankar Singh
|
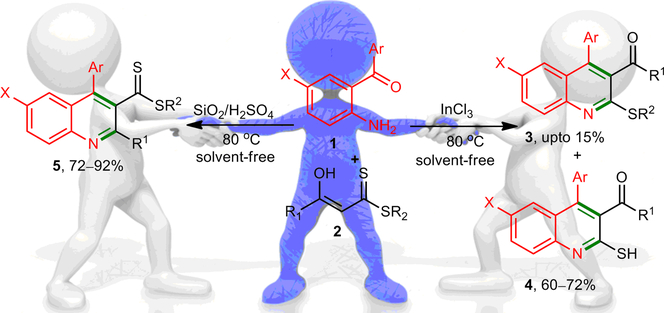
A straightforward approach for the chemodivergent synthesis of quinolines is described through site‐selective coupling of ortho‐aminoaryl ketones with α‐enolic dithioesters (DTEs) under solvent‐free conditions. The operationally and user‐simple one‐pot methodology is based on the trifunctional nature of DTEs. Both the carbonyl and the thiocarbonyl moiety in α‐enolic dithioesters were employed for the efficient construction of three differently substituted quinolines in a chemoselective manner simply by variation of an easy to handle acid catalyst.
中文翻译:

通过邻氨基芳基酮与α-烯丙基二硫代酯的位点选择性偶联,由酸控制的化学合成三种不同取代的喹啉
通过在无溶剂条件下将邻氨基芳基酮与α-烯丙基二硫代酯(DTE)进行位点选择性偶联,描述了一种喹啉化学发散合成的简单方法。操作简便,用户简单的一站式方法基于DTE的三功能性质。仅通过改变易于处理的酸催化剂,即可将α-烯丙基二硫代酸酯中的羰基和硫代羰基部分用于以化学选择性方式高效构建三种不同取代的喹啉。
更新日期:2016-03-31
中文翻译:

通过邻氨基芳基酮与α-烯丙基二硫代酯的位点选择性偶联,由酸控制的化学合成三种不同取代的喹啉
通过在无溶剂条件下将邻氨基芳基酮与α-烯丙基二硫代酯(DTE)进行位点选择性偶联,描述了一种喹啉化学发散合成的简单方法。操作简便,用户简单的一站式方法基于DTE的三功能性质。仅通过改变易于处理的酸催化剂,即可将α-烯丙基二硫代酸酯中的羰基和硫代羰基部分用于以化学选择性方式高效构建三种不同取代的喹啉。











































 京公网安备 11010802027423号
京公网安备 11010802027423号