当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Molecular Modeling, Anticonvulsant and Antinociceptive Properties of New 1,1‐Disubstituted Cyclohexane and 1,3‐Diazaspiro[4.5]decane Derivatives
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-01-25 , DOI: 10.1002/slct.201803727 Mohamed Nabil Aboul-Enein 1 , Aida A. El-Azzouny 1 , Fatma Ragab 2 , Mohammed S. Abdel-Maksoud 1 , Walaa H. Abd-Allah 3 , Yousreya Maklad 4
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-01-25 , DOI: 10.1002/slct.201803727 Mohamed Nabil Aboul-Enein 1 , Aida A. El-Azzouny 1 , Fatma Ragab 2 , Mohammed S. Abdel-Maksoud 1 , Walaa H. Abd-Allah 3 , Yousreya Maklad 4
Affiliation
|
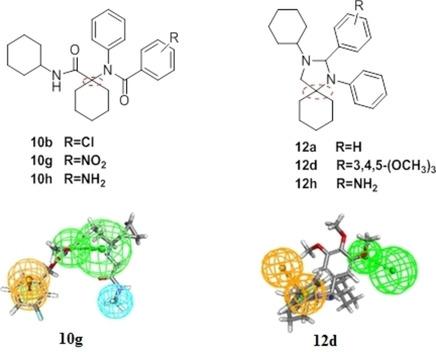
The current work describes the synthesis of new series of N‐(1‐(cyclohexylcarbamoyl) cyclohexyl)‐N‐phenylarylamides (10 a‐h) and 3‐cyclohexyl‐1,2‐diphenyl and 2‐(substituted phenyl)‐1,3‐diazaspiro[4.5]decanes (12 a‐h).The new compounds were screened for their anticonvulsant and antinociceptive activities using sodium valproate and tramadol hydrochloride, respectively as reference standards. 4‐Chloro‐N‐(1‐(cyclohexylcarbamoyl)cyclohexyl)‐N‐phenylbenzamide (10 b), 3‐Cyclohexyl‐1,2‐diphenyl‐1,3‐diazaspiro[4.5]decane (12 a) and 3‐Cyclohexyl‐1‐phenyl‐2‐(3,4,5‐trimethoxyphenyl)‐1,3‐diazaspiro[4.5]decane (12 d) showed higher anticonvulsant activity than sodium valproate at dose 0.08 mmol/kg. On the other hand, 4‐Amino‐N‐(1‐(cyclohexylcarbamoyl) cyclohexyl)‐N‐phenylbenzamide (10 h) and 3‐Cyclohexyl‐1‐phenyl‐2‐(3,4,5‐trimethoxyphenyl)‐1,3‐diazaspiro[4.5]decane (12 d) exhibited higher antinociceptive potential in both intensity and duration than tramadol hydrochloride. The pharmacokinetics of the new compounds were calculated in‐ silico. Additionally, 3D pharmacophoric model was generated.
中文翻译:

1,1-二取代环己烷和1,3-二氮杂螺[4.5]癸烷衍生物的合成,分子建模,抗惊厥和抗伤害感受特性
目前的工作描述了新系列的N-(1-(环己基氨基甲酰基)环己基)-N-苯基芳基酰胺(10 a-h)和3-环己基1,2-二苯基和2-(取代的苯基)-1的合成, 3-二氮杂螺[4.5]癸烷(12 a-h)。分别使用丙戊酸钠和盐酸曲马多作为参考标准,筛选新化合物的抗惊厥和镇痛作用。4-氯-N-(1-(环己基氨基甲酰基)环己基)-N-苯基苯甲酰胺(10 b),3-环己基-1,2-二苯基-1,3-二氮杂螺[4.5]癸烷(12 a)和3-环己基-1-苯基-2-(3,4,5-三甲氧基苯基)-1,3-二氮杂螺[4.5]癸烷(12天)在剂量为0.08 mmol / kg时,显示出比丙戊酸钠更高的抗惊厥活性。另一方面,4-氨基-N-(1-(环己基氨基甲酰基)环己基)-N-苯基苯甲酰胺(10 h)和3-环己基-1-苯基-2-(3,4,5-三甲氧基苯基)-1, 3-重氮杂螺[4.5]癸烷(12 d)在强度和持续时间方面均比盐酸曲马多具有更高的抗伤害感受能力。在计算机上计算了新化合物的药代动力学。此外,生成了3D药效团模型。
更新日期:2019-01-25
中文翻译:

1,1-二取代环己烷和1,3-二氮杂螺[4.5]癸烷衍生物的合成,分子建模,抗惊厥和抗伤害感受特性
目前的工作描述了新系列的N-(1-(环己基氨基甲酰基)环己基)-N-苯基芳基酰胺(10 a-h)和3-环己基1,2-二苯基和2-(取代的苯基)-1的合成, 3-二氮杂螺[4.5]癸烷(12 a-h)。分别使用丙戊酸钠和盐酸曲马多作为参考标准,筛选新化合物的抗惊厥和镇痛作用。4-氯-N-(1-(环己基氨基甲酰基)环己基)-N-苯基苯甲酰胺(10 b),3-环己基-1,2-二苯基-1,3-二氮杂螺[4.5]癸烷(12 a)和3-环己基-1-苯基-2-(3,4,5-三甲氧基苯基)-1,3-二氮杂螺[4.5]癸烷(12天)在剂量为0.08 mmol / kg时,显示出比丙戊酸钠更高的抗惊厥活性。另一方面,4-氨基-N-(1-(环己基氨基甲酰基)环己基)-N-苯基苯甲酰胺(10 h)和3-环己基-1-苯基-2-(3,4,5-三甲氧基苯基)-1, 3-重氮杂螺[4.5]癸烷(12 d)在强度和持续时间方面均比盐酸曲马多具有更高的抗伤害感受能力。在计算机上计算了新化合物的药代动力学。此外,生成了3D药效团模型。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号