当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
para‐TsOH‐Promoted Cascade Reaction of ortho‐Propynol Phenyl Azides for the Synthesis of 4‐Methoxy Quinolines and Propargyl Methyl Ethers: Insight on Mechanism of Propargylic Alcohols
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2019-02-12 , DOI: 10.1002/ajoc.201900047 Tao Yang 1 , Haixin Ding 1 , Ren Li 1 , Fengyan Jin 1 , Xian-Rong Song 1 , Xi Chen 1 , Jiang Bai 1 , Qiang Xiao 1 , Yong-Min Liang 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2019-02-12 , DOI: 10.1002/ajoc.201900047 Tao Yang 1 , Haixin Ding 1 , Ren Li 1 , Fengyan Jin 1 , Xian-Rong Song 1 , Xi Chen 1 , Jiang Bai 1 , Qiang Xiao 1 , Yong-Min Liang 2
Affiliation
|
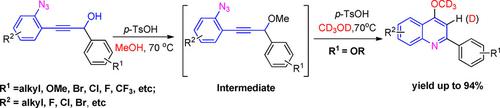
para‐TsOH‐promoted cascade reaction of easily prepared ortho‐propynol phenyl azides for the synthesis of 4‐methoxy quinolines has been developed. This reaction proceeded in a new way to generate 4‐methoxy quinolines through a concerted nucleophilic mechanism of propargyl methyl ether. It is worth noting that propargyl methyl ether can be successfully transformed into 4‐methoxy quinolines only when substrates contain a strong electron‐donating group (OR).
中文翻译:

邻苯丙酚苯叠氮化物的对-TsOH促进的级联反应,用于合成4-甲氧基喹啉和炔丙基甲基醚:炔丙醇机理的见解
已开发出对-TsOH促进的易于制备的邻丙炔丙二醇叠氮化物的级联反应,用于合成4-甲氧基喹啉。该反应以一种新的方式进行,通过炔丙基甲基醚的协同亲核机理生成4-甲氧基喹啉。值得注意的是,只有在底物包含强供电子基团(OR)时,炔丙基甲基醚才能成功转化为4-甲氧基喹啉。
更新日期:2019-02-12
中文翻译:

邻苯丙酚苯叠氮化物的对-TsOH促进的级联反应,用于合成4-甲氧基喹啉和炔丙基甲基醚:炔丙醇机理的见解
已开发出对-TsOH促进的易于制备的邻丙炔丙二醇叠氮化物的级联反应,用于合成4-甲氧基喹啉。该反应以一种新的方式进行,通过炔丙基甲基醚的协同亲核机理生成4-甲氧基喹啉。值得注意的是,只有在底物包含强供电子基团(OR)时,炔丙基甲基醚才能成功转化为4-甲氧基喹啉。































 京公网安备 11010802027423号
京公网安备 11010802027423号