当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aggregation and Tunable Color Emission Behaviors of l‐Glutamine‐Derived Platinum(II) Bipyridine Complexes by Hydrogen‐Bonding, π–π Stacking and Metal–Metal Interactions
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-03-12 , DOI: 10.1002/chem.201805901 Yeye Ai 1, 2 , Yongguang Li 1 , Heidi Li‐Ki Fu 2 , Alan Kwun‐Wa Chan 2 , Vivian Wing‐Wah Yam 1, 2
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-03-12 , DOI: 10.1002/chem.201805901 Yeye Ai 1, 2 , Yongguang Li 1 , Heidi Li‐Ki Fu 2 , Alan Kwun‐Wa Chan 2 , Vivian Wing‐Wah Yam 1, 2
Affiliation
|
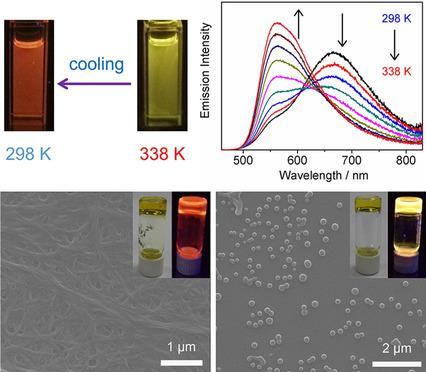
An l‐glutamine‐derived functional group was introduced to the bis(arylalkynyl)platinum(II) bipyridine complexes 1–4. The emission could be switched between the 3MLCT excited state and the triplet excimeric state through solvent or temperature changes, which is attributed to the formation and disruption of hydrogen‐bonding, π–π stacking, and metal–metal interactions. Different architectures with various morphologies, such as honeycomb nanostructures and nanospheres, were formed upon solvent variations, and these changes were accompanied by 1H NMR and distinct emission changes. Additionally, yellow and red emissive metallogels were formed at room temperature due to the different aggregation behaviors introduced by the substituent groups on bipyridine. The thermoresponsive metallogel showed emission behavior with tunable colors by controlling the temperature. The negative Gibbs free‐energy change (ΔG) and the large association constant for excimer formation have suggested that the molecules undergo aggregation through hydrogen‐bonding, π–π, and metal–metal interactions, resulting in triplet excimeric emission.
中文翻译:

氢键合,π-π堆积和金属-金属相互作用产生的l-谷氨酰胺衍生的铂(II)联吡啶配合物的聚集和可调色发射行为
甲Ñ升谷氨酰胺衍生的官能团被引入到双(芳基炔)铂(II)配合物联吡啶1 - 4。发射可以通过溶剂或温度变化在3 MLCT激发态和三重态激发态之间切换,这归因于氢键的形成和破坏,π-π堆积以及金属-金属相互作用。随着溶剂的变化,形成了具有各种形态的不同结构,例如蜂窝状纳米结构和纳米球,这些变化伴随着11 H NMR和明显的发射变化。另外,由于联吡啶上的取代基引入了不同的聚集行为,因此在室温下形成了黄色和红色的发光金属盐。通过控制温度,热响应性金属盐显示出具有可调颜色的发射行为。负的吉布斯自由能变化(ΔG)和准分子形成的大缔合常数表明,分子通过氢键,π-π和金属-金属相互作用而发生聚集,从而导致三重态激子发射。
更新日期:2019-03-12
中文翻译:

氢键合,π-π堆积和金属-金属相互作用产生的l-谷氨酰胺衍生的铂(II)联吡啶配合物的聚集和可调色发射行为
甲Ñ升谷氨酰胺衍生的官能团被引入到双(芳基炔)铂(II)配合物联吡啶1 - 4。发射可以通过溶剂或温度变化在3 MLCT激发态和三重态激发态之间切换,这归因于氢键的形成和破坏,π-π堆积以及金属-金属相互作用。随着溶剂的变化,形成了具有各种形态的不同结构,例如蜂窝状纳米结构和纳米球,这些变化伴随着11 H NMR和明显的发射变化。另外,由于联吡啶上的取代基引入了不同的聚集行为,因此在室温下形成了黄色和红色的发光金属盐。通过控制温度,热响应性金属盐显示出具有可调颜色的发射行为。负的吉布斯自由能变化(ΔG)和准分子形成的大缔合常数表明,分子通过氢键,π-π和金属-金属相互作用而发生聚集,从而导致三重态激子发射。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号