Synthesis ( IF 2.2 ) Pub Date : 2019-01-23 , DOI: 10.1055/s-0037-1611713 Xingchao Guan , Meijie Liu , Zhibo Shao , Hongpeng Li , Lu Ran , Wenling Li 1
|
Abstract
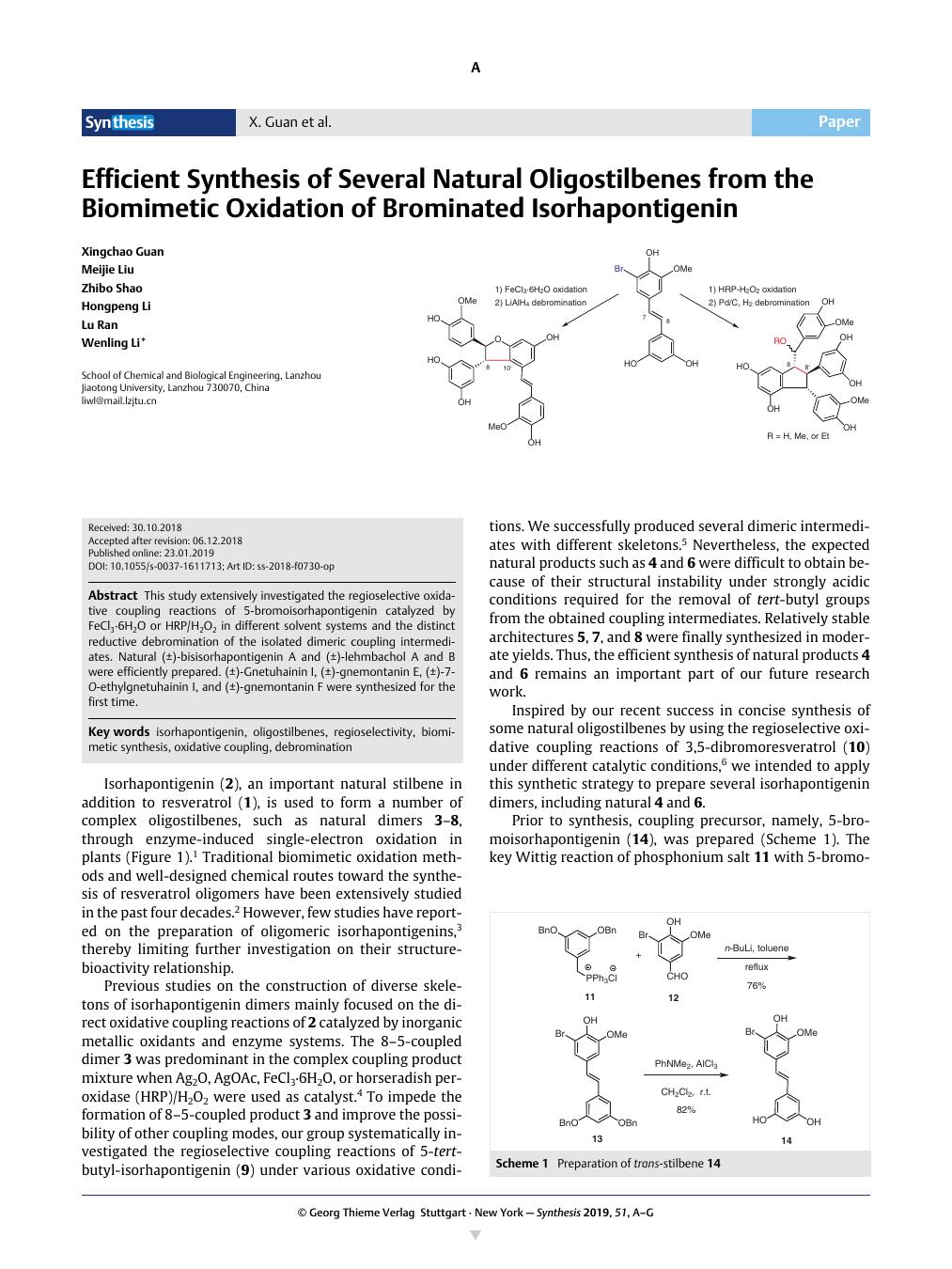
This study extensively investigated the regioselective oxidative coupling reactions of 5-bromoisorhapontigenin catalyzed by FeCl3·6H2O or HRP/H2O2 in different solvent systems and the distinct reductive debromination of the isolated dimeric coupling intermediates. Natural (±)-bisisorhapontigenin A and (±)-lehmbachol A and B were efficiently prepared. (±)-Gnetuhainin I, (±)-gnemontanin E, (±)-7-O-ethylgnetuhainin I, and (±)-gnemontanin F were synthesized for the first time.
This study extensively investigated the regioselective oxidative coupling reactions of 5-bromoisorhapontigenin catalyzed by FeCl3·6H2O or HRP/H2O2 in different solvent systems and the distinct reductive debromination of the isolated dimeric coupling intermediates. Natural (±)-bisisorhapontigenin A and (±)-lehmbachol A and B were efficiently prepared. (±)-Gnetuhainin I, (±)-gnemontanin E, (±)-7-O-ethylgnetuhainin I, and (±)-gnemontanin F were synthesized for the first time.
中文翻译:

溴化异皂甙元的仿生氧化有效合成数种天然低聚苯二酚。
摘要
这项研究广泛地研究了FeCl 3 ·6H 2 O或HRP / H 2 O 2在不同溶剂体系中催化的5-溴异佛手皂甙元的区域选择性氧化偶联反应以及分离的二聚偶合中间体的独特还原脱溴作用。天然(±)-bisisorhapontigenin A和(±)-lehmbachol A和B有效地制备。首次合成了(±)-Gnetuhainin I,(±)-gnemontanin E,(±)-7- O-乙基Gnetuhainin I和(±)-gnemontaninF。
这项研究广泛地研究了FeCl 3 ·6H 2 O或HRP / H 2 O 2在不同溶剂体系中催化的5-溴异佛手皂甙元的区域选择性氧化偶联反应以及分离的二聚偶合中间体的独特还原脱溴作用。天然(±)-bisisorhapontigenin A和(±)-lehmbachol A和B有效地制备。首次合成了(±)-Gnetuhainin I,(±)-gnemontanin E,(±)-7- O-乙基Gnetuhainin I和(±)-gnemontaninF。































 京公网安备 11010802027423号
京公网安备 11010802027423号