当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Reactivity of Aromatic Radical Cations Generated by FeCl3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-01-24 , DOI: 10.1021/jacs.8b12827 Takahiro Horibe 1 , Shuhei Ohmura 1 , Kazuaki Ishihara 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-01-24 , DOI: 10.1021/jacs.8b12827 Takahiro Horibe 1 , Shuhei Ohmura 1 , Kazuaki Ishihara 1
Affiliation
|
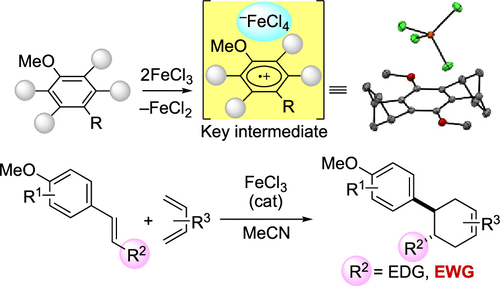
This paper describes the isolation and characterization of an aromatic radical cation generated by FeCl3. X-ray crystallographic analysis and kinetic studies reveal the mechanism of the generation of aromatic radical cation. In the solid state, a tight ion-pair of a radical cation with FeCl4- is observed. Leveraging the efficient generation of the radical cation-FeCl4- ion pair, we explore a radical cation-induced cycloaddition of trans-anethole initiated by catalytic amount of FeCl3. Both [4+2] cycloaddition and [2+2] cycloaddition with a broad substrate scope are also described. Moreover, a 100 g-scale reaction is demonstrated with the use of 1 mol % of FeCl3 as a simple and a highly active initiator.
中文翻译:

FeCl3生成的芳香族自由基阳离子的结构和反应性
本文描述了由 FeCl3 生成的芳香族自由基阳离子的分离和表征。X 射线晶体学分析和动力学研究揭示了芳香自由基阳离子的生成机制。在固态下,观察到带有 FeCl4- 的自由基阳离子的紧密离子对。利用自由基阳离子-FeCl4-离子对的有效生成,我们探索了由催化量的FeCl3引发的自由基阳离子诱导的反式茴香脑环加成反应。还描述了具有广泛底物范围的 [4+2] 环加成和 [2+2] 环加成。此外,使用 1 mol% 的 FeCl3 作为简单且高活性的引发剂,证明了 100 g 规模的反应。
更新日期:2019-01-24
中文翻译:

FeCl3生成的芳香族自由基阳离子的结构和反应性
本文描述了由 FeCl3 生成的芳香族自由基阳离子的分离和表征。X 射线晶体学分析和动力学研究揭示了芳香自由基阳离子的生成机制。在固态下,观察到带有 FeCl4- 的自由基阳离子的紧密离子对。利用自由基阳离子-FeCl4-离子对的有效生成,我们探索了由催化量的FeCl3引发的自由基阳离子诱导的反式茴香脑环加成反应。还描述了具有广泛底物范围的 [4+2] 环加成和 [2+2] 环加成。此外,使用 1 mol% 的 FeCl3 作为简单且高活性的引发剂,证明了 100 g 规模的反应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号