当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Breaking Long-Range Order in Iridium Oxide by Alkali Ion for Efficient Water Oxidation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-01-23 , DOI: 10.1021/jacs.8b11456
Jiajian Gao, Cong-Qiao Xu, Sung-Fu Hung, Wei Liu, Weizheng Cai, Zhiping Zeng, Chunmiao Jia, Hao Ming Chen, Hai Xiao, Jun Li, Yanqiang Huang, Bin Liu
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-01-23 , DOI: 10.1021/jacs.8b11456
Jiajian Gao, Cong-Qiao Xu, Sung-Fu Hung, Wei Liu, Weizheng Cai, Zhiping Zeng, Chunmiao Jia, Hao Ming Chen, Hai Xiao, Jun Li, Yanqiang Huang, Bin Liu
|
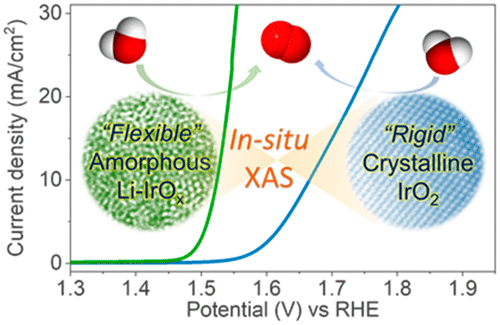
Oxygen electrochemistry plays a critical role in clean energy technologies such as fuel cells and electrolyzers, but the oxygen evolution reaction (OER) severely restricts the efficiency of these devices due to its slow kinetics. Here, we show that via incorporation of lithium ion into iridium oxide, the thus obtained amorphous iridium oxide (Li-IrO x) demonstrates outstanding water oxidation activity with an OER current density of 10 mA/cm2 at 270 mV overpotential for 10 h of continuous operation in acidic electrolyte. DFT calculations show that lithium incorporation into iridium oxide is able to lower the activation barrier for OER. X-ray absorption characterizations indicate that both amorphous Li-IrO x and rutile IrO2 own similar [IrO6] octahedron units but have different [IrO6] octahedron connection modes. Oxidation of iridium to higher oxidation states along with shrinkage in the Ir-O bond was observed by in situ X-ray absorption spectroscopy on amorphous Li-IrO x, but not on rutile IrO2 under OER operando conditions. The much more "flexible" disordered [IrO6] octahedrons with higher oxidation states in amorphous Li-IrO x as compared to the periodically interconnected "rigid" [IrO6] octahedrons in crystalline IrO2 are able to act as more electrophilic centers and thus effectively promote the fast turnover of water oxidation.
中文翻译:

用碱离子打破氧化铱中的长程有序以实现高效的水氧化
氧电化学在燃料电池和电解槽等清洁能源技术中起着至关重要的作用,但析氧反应 (OER) 由于其动力学缓慢而严重限制了这些设备的效率。在这里,我们表明,通过将锂离子掺入氧化铱中,由此获得的无定形氧化铱 (Li-IrO x) 表现出出色的水氧化活性,在 270 mV 过电位下连续 10 小时的 OER 电流密度为 10 mA/cm2。在酸性电解液中操作。DFT 计算表明,将锂掺入氧化铱能够降低 OER 的激活势垒。X 射线吸收表征表明,无定形 Li-IrO x 和金红石 IrO2 都具有相似的 [IrO6] 八面体单元,但具有不同的 [IrO6] 八面体连接模式。通过原位 X 射线吸收光谱在无定形 Li-IrO x 上观察到铱氧化为更高的氧化态以及 Ir-O 键的收缩,但在 OER 操作条件下在金红石 IrO2 上没有观察到。与晶体 IrO2 中周期性互连的“刚性”[IrO6] 八面体相比,非晶态 Li-IrO x 中具有更高氧化态的更“灵活”的无序 [IrO6] 八面体能够充当更多的亲电子中心,从而有效地促进水氧化的快速周转。
更新日期:2019-01-23
中文翻译:

用碱离子打破氧化铱中的长程有序以实现高效的水氧化
氧电化学在燃料电池和电解槽等清洁能源技术中起着至关重要的作用,但析氧反应 (OER) 由于其动力学缓慢而严重限制了这些设备的效率。在这里,我们表明,通过将锂离子掺入氧化铱中,由此获得的无定形氧化铱 (Li-IrO x) 表现出出色的水氧化活性,在 270 mV 过电位下连续 10 小时的 OER 电流密度为 10 mA/cm2。在酸性电解液中操作。DFT 计算表明,将锂掺入氧化铱能够降低 OER 的激活势垒。X 射线吸收表征表明,无定形 Li-IrO x 和金红石 IrO2 都具有相似的 [IrO6] 八面体单元,但具有不同的 [IrO6] 八面体连接模式。通过原位 X 射线吸收光谱在无定形 Li-IrO x 上观察到铱氧化为更高的氧化态以及 Ir-O 键的收缩,但在 OER 操作条件下在金红石 IrO2 上没有观察到。与晶体 IrO2 中周期性互连的“刚性”[IrO6] 八面体相比,非晶态 Li-IrO x 中具有更高氧化态的更“灵活”的无序 [IrO6] 八面体能够充当更多的亲电子中心,从而有效地促进水氧化的快速周转。

































 京公网安备 11010802027423号
京公网安备 11010802027423号