当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper amine oxidases catalyze the oxidative deamination and hydrolysis of cyclic imines.
Nature Communications ( IF 14.7 ) Pub Date : 2019-01-24 , DOI: 10.1038/s41467-018-08280-w Toshiki Nagakubo 1 , Takuto Kumano 1 , Takehiro Ohta 2, 3 , Yoshiteru Hashimoto 1 , Michihiko Kobayashi 1
Nature Communications ( IF 14.7 ) Pub Date : 2019-01-24 , DOI: 10.1038/s41467-018-08280-w Toshiki Nagakubo 1 , Takuto Kumano 1 , Takehiro Ohta 2, 3 , Yoshiteru Hashimoto 1 , Michihiko Kobayashi 1
Affiliation
|
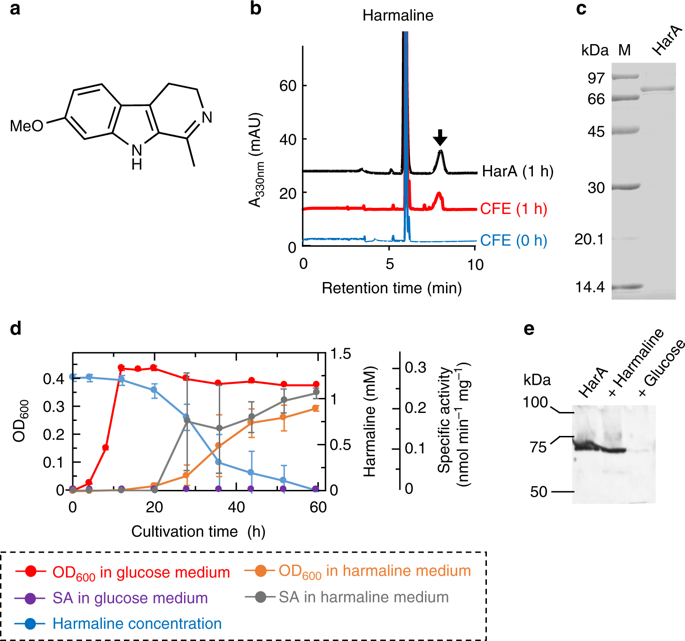
Although cyclic imines are present in various bioactive secondary metabolites, their degradative metabolism remains unknown. Here, we report that copper amine oxidases, which are important in metabolism of primary amines, catalyze a cyclic imine cleavage reaction. We isolate a microorganism (Arthrobacter sp. C-4A) which metabolizes a β-carboline alkaloid, harmaline. The harmaline-metabolizing enzyme (HarA) purified from strain C-4A is found to be copper amine oxidase and catalyze a ring-opening reaction of cyclic imine within harmaline, besides oxidative deamination of amines. Growth experiments on strain C-4A and Western blot analysis indicate that the HarA expression is induced by harmaline. We propose a reaction mechanism of the cyclic imine cleavage by HarA containing a post-translationally-synthesized cofactor, topaquinone. Together with the above results, the finding of the same activity of copper amine oxidase from E. coli suggests that, in many living organisms, these enzymes may play crucial roles in metabolism of ubiquitous cyclic imines.
中文翻译:

铜胺氧化酶催化环状亚胺的氧化脱氨和水解。
尽管环状亚胺存在于各种具有生物活性的次生代谢产物中,但它们的降解代谢仍然未知。在这里,我们报告铜胺氧化酶,这在伯胺的代谢中很重要,它催化环状亚胺裂解反应。我们分离出一种可代谢β-咔啉生物碱harmaline的微生物(Arthrobacter sp。C-4A)。发现从菌株C-4A纯化的harmaline代谢酶(HarA)是铜胺氧化酶,除了胺的氧化脱氨作用外,还催化harmaline中环状亚胺的开环反应。在菌株C-4A上的生长实验和Western印迹分析表明HarA表达是由harmaline诱导的。我们提出了含有翻译后合成的辅因子托帕醌的HarA切割亚胺的反应机理。
更新日期:2019-01-24
中文翻译:

铜胺氧化酶催化环状亚胺的氧化脱氨和水解。
尽管环状亚胺存在于各种具有生物活性的次生代谢产物中,但它们的降解代谢仍然未知。在这里,我们报告铜胺氧化酶,这在伯胺的代谢中很重要,它催化环状亚胺裂解反应。我们分离出一种可代谢β-咔啉生物碱harmaline的微生物(Arthrobacter sp。C-4A)。发现从菌株C-4A纯化的harmaline代谢酶(HarA)是铜胺氧化酶,除了胺的氧化脱氨作用外,还催化harmaline中环状亚胺的开环反应。在菌株C-4A上的生长实验和Western印迹分析表明HarA表达是由harmaline诱导的。我们提出了含有翻译后合成的辅因子托帕醌的HarA切割亚胺的反应机理。































 京公网安备 11010802027423号
京公网安备 11010802027423号