Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Autophagic cell death restricts chromosomal instability during replicative crisis
Nature ( IF 50.5 ) Pub Date : 2019-01-01 , DOI: 10.1038/s41586-019-0885-0 Joe Nassour 1 , Robert Radford 1 , Adriana Correia 1 , Javier Miralles Fusté 1 , Brigitte Schoell 2 , Anna Jauch 2 , Reuben J Shaw 1 , Jan Karlseder 1
Nature ( IF 50.5 ) Pub Date : 2019-01-01 , DOI: 10.1038/s41586-019-0885-0 Joe Nassour 1 , Robert Radford 1 , Adriana Correia 1 , Javier Miralles Fusté 1 , Brigitte Schoell 2 , Anna Jauch 2 , Reuben J Shaw 1 , Jan Karlseder 1
Affiliation
|
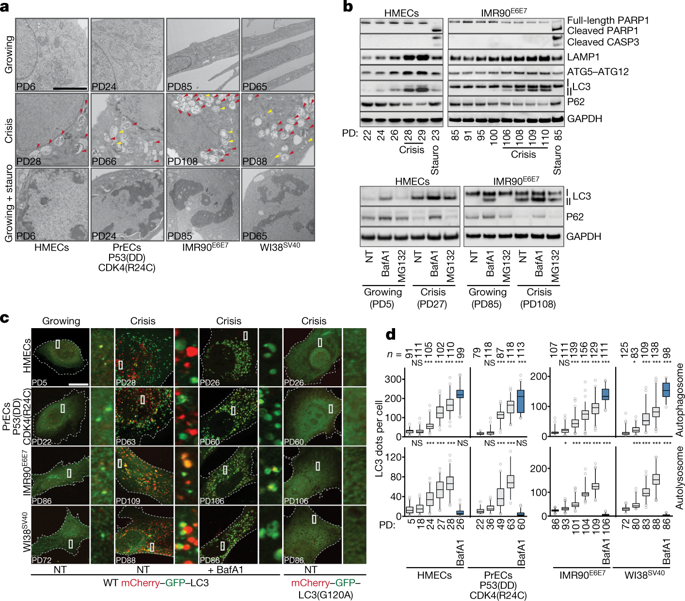
Replicative crisis is a senescence-independent process that acts as a final barrier against oncogenic transformation by eliminating pre-cancerous cells with disrupted cell cycle checkpoints1. It functions as a potent tumour suppressor and culminates in extensive cell death. Cells rarely evade elimination and evolve towards malignancy, but the mechanisms that underlie cell death in crisis are not well understood. Here we show that macroautophagy has a dominant role in the death of fibroblasts and epithelial cells during crisis. Activation of autophagy is critical for cell death, as its suppression promoted bypass of crisis, continued proliferation and accumulation of genome instability. Telomere dysfunction specifically triggers autophagy, implicating a telomere-driven autophagy pathway that is not induced by intrachromosomal breaks. Telomeric DNA damage generates cytosolic DNA species with fragile nuclear envelopes that undergo spontaneous disruption. The cytosolic chromatin fragments activate the cGAS–STING (cyclic GMP-AMP synthase–stimulator of interferon genes) pathway and engage the autophagy machinery. Our data suggest that autophagy is an integral component of the tumour suppressive crisis mechanism and that loss of autophagy function is required for the initiation of cancer.Cell death during replicative crisis involves autophagy induced by telomere dysfunction.
中文翻译:

自噬细胞死亡限制复制危机期间染色体的不稳定性
复制危机是一个独立于衰老的过程,通过消除细胞周期检查点被破坏的癌前细胞,充当致癌转化的最终屏障。它作为一种有效的肿瘤抑制剂发挥作用,并最终导致广泛的细胞死亡。细胞很少能逃避消除并进化为恶性肿瘤,但危机中细胞死亡的机制尚不清楚。在这里,我们表明巨自噬在危机期间成纤维细胞和上皮细胞的死亡中起主导作用。自噬的激活对于细胞死亡至关重要,因为它的抑制促进了危机的绕过、持续增殖和基因组不稳定性的积累。端粒功能障碍特异性触发自噬,这表明端粒驱动的自噬途径不是由染色体内断裂诱导的。端粒 DNA 损伤会产生胞浆 DNA 物质,其核膜易碎,会发生自发破坏。胞质染色质片段激活 cGAS-STING(环 GMP-AMP 合酶 - 干扰素基因刺激剂)途径并参与自噬机制。我们的数据表明,自噬是肿瘤抑制危机机制的一个组成部分,自噬功能的丧失是癌症发生所必需的。复制危机期间的细胞死亡涉及端粒功能障碍诱导的自噬。
更新日期:2019-01-01
中文翻译:

自噬细胞死亡限制复制危机期间染色体的不稳定性
复制危机是一个独立于衰老的过程,通过消除细胞周期检查点被破坏的癌前细胞,充当致癌转化的最终屏障。它作为一种有效的肿瘤抑制剂发挥作用,并最终导致广泛的细胞死亡。细胞很少能逃避消除并进化为恶性肿瘤,但危机中细胞死亡的机制尚不清楚。在这里,我们表明巨自噬在危机期间成纤维细胞和上皮细胞的死亡中起主导作用。自噬的激活对于细胞死亡至关重要,因为它的抑制促进了危机的绕过、持续增殖和基因组不稳定性的积累。端粒功能障碍特异性触发自噬,这表明端粒驱动的自噬途径不是由染色体内断裂诱导的。端粒 DNA 损伤会产生胞浆 DNA 物质,其核膜易碎,会发生自发破坏。胞质染色质片段激活 cGAS-STING(环 GMP-AMP 合酶 - 干扰素基因刺激剂)途径并参与自噬机制。我们的数据表明,自噬是肿瘤抑制危机机制的一个组成部分,自噬功能的丧失是癌症发生所必需的。复制危机期间的细胞死亡涉及端粒功能障碍诱导的自噬。















































 京公网安备 11010802027423号
京公网安备 11010802027423号