当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Chiral Piperidines via the Stepwise Dearomatization/Borylation of Pyridines
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-03-28 , DOI: 10.1021/jacs.6b01375 Koji Kubota 1 , Yuta Watanabe 1 , Keiichi Hayama 1 , Hajime Ito 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-03-28 , DOI: 10.1021/jacs.6b01375 Koji Kubota 1 , Yuta Watanabe 1 , Keiichi Hayama 1 , Hajime Ito 1
Affiliation
|
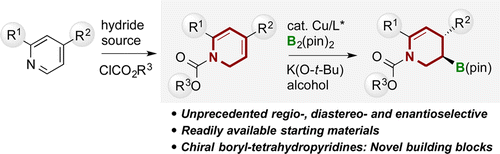
We have developed a novel approach for the synthesis of enantioenriched 3-boryl-tetrahydropyridines via the Cu(I)-catalyzed regio-, diastereo-, and enantioselective protoborylation of 1,2-dihydropyridines, which were obtained by the partial reduction of the pyridine derivatives. This dearomatization/enantioselective borylation stepwise strategy provides facile access to chiral piperidines together with the stereospecific transformation of a stereogenic C-B bond from readily available starting materials. Furthermore, the utility of this method is demonstrated for the concise synthesis of the antidepressant drug (-)-paroxetine. A theoretical study of the reaction mechanism is also described.
中文翻译:

通过吡啶的逐步脱芳构化/硼酸化反应选择性合成手性哌啶
我们开发了一种通过 Cu(I) 催化的 1,2-二氢吡啶的区域选择性、非对映选择性和对映选择性原硼化反应合成富含对映体的 3-硼基四氢吡啶的新方法,该方法是通过部分还原吡啶获得的衍生品。这种脱芳构化/对映选择性硼酸化逐步策略提供了轻松获得手性哌啶以及从容易获得的起始材料中立体定向 CB 键的立体有择转化的途径。此外,该方法在抗抑郁药物 (-)-帕罗西汀的简明合成方面的实用性得到了证明。还描述了反应机理的理论研究。
更新日期:2016-03-28
中文翻译:

通过吡啶的逐步脱芳构化/硼酸化反应选择性合成手性哌啶
我们开发了一种通过 Cu(I) 催化的 1,2-二氢吡啶的区域选择性、非对映选择性和对映选择性原硼化反应合成富含对映体的 3-硼基四氢吡啶的新方法,该方法是通过部分还原吡啶获得的衍生品。这种脱芳构化/对映选择性硼酸化逐步策略提供了轻松获得手性哌啶以及从容易获得的起始材料中立体定向 CB 键的立体有择转化的途径。此外,该方法在抗抑郁药物 (-)-帕罗西汀的简明合成方面的实用性得到了证明。还描述了反应机理的理论研究。































 京公网安备 11010802027423号
京公网安备 11010802027423号