当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Selective Synthesis of 2-(2H-1,2,3-Triazol-2-yl)benzoic Acids
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-01-18 00:00:00 , DOI: 10.1021/acs.oprd.8b00349 Remo Roth 1 , Gunther Schmidt 1 , Alice Prud’homme 1 , Stefan Abele 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-01-18 00:00:00 , DOI: 10.1021/acs.oprd.8b00349 Remo Roth 1 , Gunther Schmidt 1 , Alice Prud’homme 1 , Stefan Abele 1
Affiliation
|
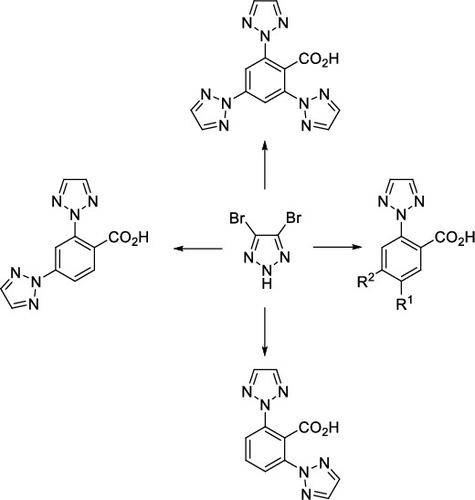
A selective and scalable synthesis of 2-(2H-1,2,3-triazol-2-yl)benzoic acid starting from 1-fluoro-2-nitrobenzene derivatives is presented. The four-step synthesis introduces the triazole at the start via N2-arylation of 4,5-dibromo-2H-1,2,3-triazole. A sequence of consecutive functional group transformations, namely hydrogenation, Sandmeyer iodination, and Grignard carboxylation, provides the target molecules in a reliable and scalable manner. The usefulness of this method is demonstrated by the synthesis of di- or tri(2H-1,2,3-triazol-2-yl)benzene derivatives, which are difficult to produce by other methods.
中文翻译:

2-(2 H -1,2,3-三唑-2-基)苯甲酸的高选择性合成
提出了从1-氟-2-硝基苯衍生物开始的选择性和可扩展的2-(2 H -1,2,3-三唑-2-基)苯甲酸的合成。四步合成通过4,5-二溴-2 H -1,2,3-三唑的N 2-芳基化在开始时引入三唑。一系列连续的官能团转化,即氢化,桑德迈碘化和格利雅(Grignard)羧化反应,以可靠且可扩展的方式提供了目标分子。通过合成难以用其他方法生产的二或三(2 H -1,2,3-三唑-2-基)苯衍生物证明了该方法的有效性。
更新日期:2019-01-18
中文翻译:

2-(2 H -1,2,3-三唑-2-基)苯甲酸的高选择性合成
提出了从1-氟-2-硝基苯衍生物开始的选择性和可扩展的2-(2 H -1,2,3-三唑-2-基)苯甲酸的合成。四步合成通过4,5-二溴-2 H -1,2,3-三唑的N 2-芳基化在开始时引入三唑。一系列连续的官能团转化,即氢化,桑德迈碘化和格利雅(Grignard)羧化反应,以可靠且可扩展的方式提供了目标分子。通过合成难以用其他方法生产的二或三(2 H -1,2,3-三唑-2-基)苯衍生物证明了该方法的有效性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号