当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Release, Separation, and Recovery of Monomeric Reducing N-Glycans with Pronase E Combined with 9-Chloromethyl Chloroformate and Glycosylasparaginase
Biochemistry ( IF 2.9 ) Pub Date : 2019-01-19 00:00:00 , DOI: 10.1021/acs.biochem.8b01224
Yu Lu 1 , Cheng Li 1 , Ming Wei 1 , Yue Jia 1 , Jingjing Song 1 , Ying Zhang 1 , Chengjian Wang 1, 2 , Linjuan Huang 1, 2 , Zhongfu Wang 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2019-01-19 00:00:00 , DOI: 10.1021/acs.biochem.8b01224
Yu Lu 1 , Cheng Li 1 , Ming Wei 1 , Yue Jia 1 , Jingjing Song 1 , Ying Zhang 1 , Chengjian Wang 1, 2 , Linjuan Huang 1, 2 , Zhongfu Wang 1, 2
Affiliation
|
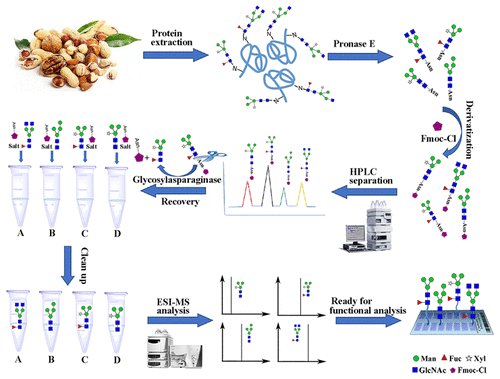
The glycan moiety of glycoproteins plays key roles in various biological processes. However, there are few versatile methods for releasing, separating, and recovering monomeric reducing N-glycans for further functional analysis. In this study, we developed a new method to achieve the release, separation, and recovery of monomeric reducing N-glycans using enzyme E (Pronase E) combined with 9-chloromethyl chloroformate (Fmoc-Cl) and glycosylasparaginase (GA). Ovalbumin, ribonuclease B, ginkgo, and pine nut glycoproteins were used as materials and sequentially enzymatically hydrolyzed with Pronase E, derivatized with Fmoc-Cl, and enzymatically hydrolyzed with GA. The products produced by this method were then detected by electrospray ionization mass spectrometry, high-performance liquid chromatography (HPLC), and online hydrophilic interaction chromatography (HILIC-MS) separation. The results showed that all N-glycans with essentially one amino acid obtained with Pronase E were labeled with Fmoc-Cl and could be efficiently separated and detected via HPLC and HILIC-MS. Finally, the isolated Asn-glycan derivatives were digested with GA, enabling the recovery of all monomeric reducing N-glycans modified by core α-1,3 fucose. This method was simple, inexpensive, and broadly applicable and could therefore be quite important for analysis of the structure–function relationships of glycans.
中文翻译:

链霉蛋白酶E与9-氯甲基氯甲酸酯和糖基葡聚糖酶联用的单体还原性N-聚糖的释放,分离和回收
糖蛋白的聚糖部分在各种生物学过程中起关键作用。然而,几乎没有用于释放,分离和回收单体还原性N-聚糖用于进一步功能分析的通用方法。在这项研究中,我们开发了一种新的方法,可以使用酶E(Pronase E)与9-氯甲基氯甲酸酯(Fmoc-Cl)和糖基葡聚糖酶(GA)结合来实现单体还原性N-聚糖的释放,分离和回收。使用卵清蛋白,核糖核酸酶B,银杏和松子糖蛋白作为原料,依次用Pronase E酶解,用Fmoc-Cl衍生化和用GA酶解。然后通过电喷雾电离质谱,高效液相色谱(HPLC)检测通过这种方法生产的产物,以及在线亲水相互作用色谱(HILIC-MS)分离。结果表明,所有用Pronase E获得的基本上具有一个氨基酸的N-聚糖都用Fmoc-Cl标记,并且可以通过HPLC和HILIC-MS进行有效分离和检测。最后,用GA消化分离出的Asn-聚糖衍生物,从而回收由核心α-1,3果糖修饰的所有单体还原N-聚糖。该方法简单,便宜且可广泛应用,因此对于分析聚糖的结构-功能关系可能非常重要。分离的Asn-聚糖衍生物用GA消化,可以回收所有被核心α-1,3果糖修饰的单体还原性N-聚糖。该方法简单,便宜且可广泛应用,因此对于分析聚糖的结构-功能关系可能非常重要。分离的Asn-聚糖衍生物用GA消化,可以回收所有被核心α-1,3果糖修饰的单体还原性N-聚糖。该方法简单,便宜且可广泛应用,因此对于分析聚糖的结构-功能关系可能非常重要。
更新日期:2019-01-19
中文翻译:

链霉蛋白酶E与9-氯甲基氯甲酸酯和糖基葡聚糖酶联用的单体还原性N-聚糖的释放,分离和回收
糖蛋白的聚糖部分在各种生物学过程中起关键作用。然而,几乎没有用于释放,分离和回收单体还原性N-聚糖用于进一步功能分析的通用方法。在这项研究中,我们开发了一种新的方法,可以使用酶E(Pronase E)与9-氯甲基氯甲酸酯(Fmoc-Cl)和糖基葡聚糖酶(GA)结合来实现单体还原性N-聚糖的释放,分离和回收。使用卵清蛋白,核糖核酸酶B,银杏和松子糖蛋白作为原料,依次用Pronase E酶解,用Fmoc-Cl衍生化和用GA酶解。然后通过电喷雾电离质谱,高效液相色谱(HPLC)检测通过这种方法生产的产物,以及在线亲水相互作用色谱(HILIC-MS)分离。结果表明,所有用Pronase E获得的基本上具有一个氨基酸的N-聚糖都用Fmoc-Cl标记,并且可以通过HPLC和HILIC-MS进行有效分离和检测。最后,用GA消化分离出的Asn-聚糖衍生物,从而回收由核心α-1,3果糖修饰的所有单体还原N-聚糖。该方法简单,便宜且可广泛应用,因此对于分析聚糖的结构-功能关系可能非常重要。分离的Asn-聚糖衍生物用GA消化,可以回收所有被核心α-1,3果糖修饰的单体还原性N-聚糖。该方法简单,便宜且可广泛应用,因此对于分析聚糖的结构-功能关系可能非常重要。分离的Asn-聚糖衍生物用GA消化,可以回收所有被核心α-1,3果糖修饰的单体还原性N-聚糖。该方法简单,便宜且可广泛应用,因此对于分析聚糖的结构-功能关系可能非常重要。







































 京公网安备 11010802027423号
京公网安备 11010802027423号