当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conversion of Aminodeoxychorismate Synthase into Anthranilate Synthase with Janus Mutations: Mechanism of Pyruvate Elimination Catalyzed by Chorismate Enzymes
Biochemistry ( IF 2.9 ) Pub Date : 2015-04-02 00:00:00 , DOI: 10.1021/acs.biochem.5b00013 Justin E. Culbertson 1 , Dong hee Chung 1 , Kristin T. Ziebart 2 , Eduardo Espiritu 3 , Michael D. Toney 1
Biochemistry ( IF 2.9 ) Pub Date : 2015-04-02 00:00:00 , DOI: 10.1021/acs.biochem.5b00013 Justin E. Culbertson 1 , Dong hee Chung 1 , Kristin T. Ziebart 2 , Eduardo Espiritu 3 , Michael D. Toney 1
Affiliation
|
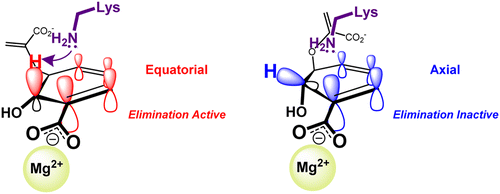
The central importance of chorismate enzymes in bacteria, fungi, parasites, and plants combined with their absence in mammals makes them attractive targets for antimicrobials and herbicides. Two of these enzymes, anthranilate synthase (AS) and aminodeoxychorismate synthase (ADCS), are structurally and mechanistically similar. The first catalytic step, amination at C2, is common between them, but AS additionally catalyzes pyruvate elimination, aromatizing the aminated intermediate to anthranilate. Despite prior attempts, the conversion of a pyruvate elimination-deficient enzyme into an elimination-proficient one has not been reported. Janus, a bioinformatics method for predicting mutations required to functionally interconvert homologous enzymes, was employed to predict mutations to convert ADCS into AS. A genetic selection on a library of Janus-predicted mutations was performed. Complementation of an AS-deficient strain of Escherichia coli grown on minimal medium led to several ADCS mutants that allow growth in 6 days compared to 2 days for wild-type AS. The purified mutant enzymes catalyze the conversion of chorismate to anthranilate at rates that are ∼50% of the rate of wild-type ADCS-catalyzed conversion of chorismate to aminodeoxychorismate. The residues mutated do not contact the substrate. Molecular dynamics studies suggest that pyruvate elimination is controlled by the conformation of the C2-aminated intermediate. Enzymes that catalyze elimination favor the equatorial conformation, which presents the C2-H to a conserved active site lysine (Lys424) for deprotonation and maximizes stereoelectronic activation. Acid/base catalysis of pyruvate elimination was confirmed in AS and salicylate synthase by showing incorporation of a solvent-derived proton into the pyruvate methyl group and by solvent kinetic isotope effects on pyruvate elimination catalyzed by AS.
中文翻译:

带有Janus突变的氨基去氧胆酸合酶转变为邻氨基苯甲酸合酶:分支酸酶催化丙酮酸消除的机理。
分支酸酶在细菌,真菌,寄生虫和植物中的重要地位,再加上它们在哺乳动物中的缺失,使其成为抗菌剂和除草剂的有吸引力的靶标。这些酶中的两种,邻氨基苯甲酸合酶(AS)和氨基脱氧胆酸合酶(ADCS),在结构和机理上相似。第一步是在C2上进行胺化,这是它们之间的共同步骤,但AS还催化丙酮酸的消除,将胺化的中间体芳构化为邻氨基苯甲酸酯。尽管有先前的尝试,但尚未报道丙酮酸盐消除缺陷型酶向消除缺陷型酶的转化。Janus是一种生物信息学方法,用于预测功能上相互转换同源酶所需的突变,该方法用于预测将ADCS转换为AS的突变。在Janus预测的突变库中进行了遗传选择。缺乏AS的菌株的补充大肠杆菌在基本培养基上生长会导致几种ADCS突变体,这些突变体可在6天之内生长,而野生型AS则需要2天。纯化的突变酶催化分支酸转化为邻氨基苯甲酸的速率约为野生型ADCS催化分支酸转化为氨基脱氧胆酸酯的速率的50%。突变的残基不接触底物。分子动力学研究表明,丙酮酸的消除受C2胺化中间体的构象控制。催化消除的酶偏爱赤道构象,该构象将C2-H呈现给保守的活性位点赖氨酸(Lys424)进行去质子化并最大化立体电子激活。
更新日期:2015-04-02
中文翻译:

带有Janus突变的氨基去氧胆酸合酶转变为邻氨基苯甲酸合酶:分支酸酶催化丙酮酸消除的机理。
分支酸酶在细菌,真菌,寄生虫和植物中的重要地位,再加上它们在哺乳动物中的缺失,使其成为抗菌剂和除草剂的有吸引力的靶标。这些酶中的两种,邻氨基苯甲酸合酶(AS)和氨基脱氧胆酸合酶(ADCS),在结构和机理上相似。第一步是在C2上进行胺化,这是它们之间的共同步骤,但AS还催化丙酮酸的消除,将胺化的中间体芳构化为邻氨基苯甲酸酯。尽管有先前的尝试,但尚未报道丙酮酸盐消除缺陷型酶向消除缺陷型酶的转化。Janus是一种生物信息学方法,用于预测功能上相互转换同源酶所需的突变,该方法用于预测将ADCS转换为AS的突变。在Janus预测的突变库中进行了遗传选择。缺乏AS的菌株的补充大肠杆菌在基本培养基上生长会导致几种ADCS突变体,这些突变体可在6天之内生长,而野生型AS则需要2天。纯化的突变酶催化分支酸转化为邻氨基苯甲酸的速率约为野生型ADCS催化分支酸转化为氨基脱氧胆酸酯的速率的50%。突变的残基不接触底物。分子动力学研究表明,丙酮酸的消除受C2胺化中间体的构象控制。催化消除的酶偏爱赤道构象,该构象将C2-H呈现给保守的活性位点赖氨酸(Lys424)进行去质子化并最大化立体电子激活。































 京公网安备 11010802027423号
京公网安备 11010802027423号