当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of the Binding Site of Apical Membrane Antigen 1 (AMA1) Inhibitors Using a Paramagnetic Probe.
ChemMedChem ( IF 3.6 ) Pub Date : 2019-02-13 , DOI: 10.1002/cmdc.201800802
Mansura Akter 1 , Nyssa Drinkwater 2 , Shane M Devine 1 , Simon C Drew 3 , Bankala Krishnarjuna 1 , Cael O Debono 1 , Geqing Wang 1 , Martin J Scanlon 1 , Peter J Scammells 1 , Sheena McGowan 2 , Christopher A MacRaild 1 , Raymond S Norton 1
ChemMedChem ( IF 3.6 ) Pub Date : 2019-02-13 , DOI: 10.1002/cmdc.201800802
Mansura Akter 1 , Nyssa Drinkwater 2 , Shane M Devine 1 , Simon C Drew 3 , Bankala Krishnarjuna 1 , Cael O Debono 1 , Geqing Wang 1 , Martin J Scanlon 1 , Peter J Scammells 1 , Sheena McGowan 2 , Christopher A MacRaild 1 , Raymond S Norton 1
Affiliation
|
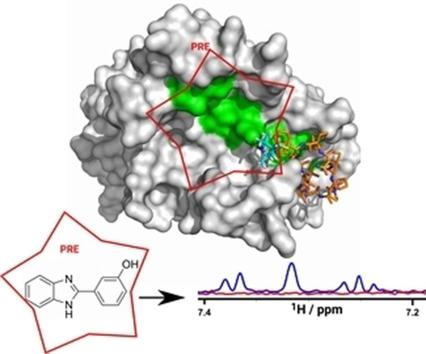
Apical membrane antigen 1 (AMA1) is essential for the invasion of host cells by malaria parasites. Several small-molecule ligands have been shown to bind to a conserved hydrophobic cleft in Plasmodium falciparum AMA1. However, a lack of detailed structural information on the binding pose of these molecules has hindered their further optimisation as inhibitors. We have developed a spin-labelled peptide based on RON2, the native binding partner of AMA1, to probe the binding sites of compounds on PfAMA1. The crystal structure of this peptide bound to PfAMA1 shows that it binds at one end of the hydrophobic groove, leaving much of the binding site unoccupied and allowing fragment hits to bind without interference. In paramagnetic relaxation enhancement (PRE)-based NMR screening, the 1 H relaxation rates of compounds binding close to the probe were enhanced. Compounds experienced different degrees of PRE as a result of their different orientations relative to the spin label while bound to AMA1. Thus, PRE-derived distance constraints can be used to identify binding sites and guide further hit optimisation.
中文翻译:

使用顺磁探针鉴定顶膜抗原1(AMA1)抑制剂的结合位点。
顶端膜抗原1(AMA1)对于疟疾寄生虫入侵宿主细胞至关重要。几种小分子配体已显示与恶性疟原虫AMA1中保守的疏水裂隙结合。然而,关于这些分子的结合姿势缺乏详细的结构信息阻碍了它们作为抑制剂的进一步优化。我们已经开发了一种基于RON2(AMA1的天然结合伴侣)的自旋标记肽,以探测PfAMA1上化合物的结合位点。与PfAMA1结合的这种肽的晶体结构表明,它在疏水槽的一端结合,从而使许多结合位点未被占用,并使片段命中结合而不受干扰。在基于顺磁弛豫增强(PRE)的NMR筛选中,化合物与探针结合的1 H弛豫速率提高。由于与AMA1结合时相对于自旋标记的取向不同,化合物的PRE程度不同。因此,PRE衍生的距离约束可用于识别结合位点并指导进一步的命中优化。
更新日期:2019-02-13
中文翻译:

使用顺磁探针鉴定顶膜抗原1(AMA1)抑制剂的结合位点。
顶端膜抗原1(AMA1)对于疟疾寄生虫入侵宿主细胞至关重要。几种小分子配体已显示与恶性疟原虫AMA1中保守的疏水裂隙结合。然而,关于这些分子的结合姿势缺乏详细的结构信息阻碍了它们作为抑制剂的进一步优化。我们已经开发了一种基于RON2(AMA1的天然结合伴侣)的自旋标记肽,以探测PfAMA1上化合物的结合位点。与PfAMA1结合的这种肽的晶体结构表明,它在疏水槽的一端结合,从而使许多结合位点未被占用,并使片段命中结合而不受干扰。在基于顺磁弛豫增强(PRE)的NMR筛选中,化合物与探针结合的1 H弛豫速率提高。由于与AMA1结合时相对于自旋标记的取向不同,化合物的PRE程度不同。因此,PRE衍生的距离约束可用于识别结合位点并指导进一步的命中优化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号