当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanosheets of Nickel Iron Hydroxy Carbonate Hydrate with Pronounced OER Activity under Alkaline and Near-Neutral Conditions
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-01-16 00:00:00 , DOI: 10.1021/acs.inorgchem.8b02680 Kannimuthu Karthick 1, 2 , Sengeni Anantharaj 1, 2 , Sivasankara Rao Ede 1, 2 , Subrata Kundu 1, 2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-01-16 00:00:00 , DOI: 10.1021/acs.inorgchem.8b02680 Kannimuthu Karthick 1, 2 , Sengeni Anantharaj 1, 2 , Sivasankara Rao Ede 1, 2 , Subrata Kundu 1, 2
Affiliation
|
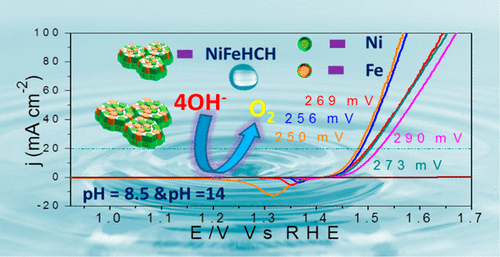
Evaluation of unique catalysts of the iron group metals with activity in the OER region similar to that of scarce metals is of great importance to achieve sustainable production of H2 on a large scale. Herein, we report the unique nanosheets of nickel iron hydroxy carbonate hydrate (NiFeHCH) which were prepared through a wet-chemical route within 1 h of reaction time, acting as an efficient electrocatalyst for the oxygen evolution reaction (OER) in both alkaline and near-neutral media. The NiFeHCH was prepared with different concentrations of Fe in different ratios: 1:0.2, 1:0.4, 1:0.6, 1:0.8, and 1:1. Among them, nanosheets of NiFeHCH (1:0.2) were found to have superior OER activity, which required an overpotential of 250 mV to reach 20 mA cm–2 with a very low Tafel slope value of 39 mV/decade in 1 M KOH. Nanosheets with other ratios also had comparable OER activity with overpotentials ranging from 256 to 290 mV with Tafel slope values from 39 to 49 mV/decade. Nanosheets of NiFeHCH electrocatalysts screened for the OER in 1 M NaHCO3 (pH ∼8.5) required overpotentials for all of the ratios ranging from 389 to 507 mV at 10 mA cm–2 and Tafel slope values from 155 to 205 mV/decade, of which nanosheets of NiFeHCH (1:0.4) showed better activity by requiring an overpotential of 389 mV at 10 mA cm–2 and Tafel slope value of 155 mV/decade. With these fruitful advantages, these prepared nanosheets of NiFeHCH can be a better alternative to scarce metals for industrial water electrolysis.
中文翻译:

碱性和近中性条件下具有显着OER活性的镍铁羟基碳酸盐水合物的纳米片
评估在OER地区具有与稀有金属相似的活性的铁族金属的独特催化剂对于实现大规模的H 2可持续生产非常重要。在此,我们报道了在反应时间1小时内通过湿化学方法制备的羟基碳酸镍铁水合物(NiFeHCH)的独特纳米片,在碱性和近氧化氢反应中作为氧释放反应(OER)的有效电催化剂-中性媒体。用不同比例的不同浓度的Fe制备NiFeHCH:1:0.2、1:0.4、1:0.6、1:0.8和1:1。其中,发现NiFeHCH(1:0.2)纳米片具有优异的OER活性,需要250 mV的超电势才能达到20 mA cm –2在1 M KOH中具有非常低的Tafel斜率值,即39 mV /十倍频程。具有其他比率的纳米片也具有相当的OER活性,超电势的范围为256至290 mV,Tafel斜率值为39至49 mV /十倍。在1 M NaHCO 3(pH〜8.5)中针对OER筛选的NiFeHCH电催化剂纳米片需要在10 mA cm –2的所有比率范围从389至507 mV和Tafel斜率值从155至205 mV /十年的所有条件下的超电势。 NiFeHCH(1:0.4)纳米片表现出更好的活性,因为在10 mA cm –2时需要389 mV的过电势,而Tafel斜率值为155 mV /十年。凭借这些卓有成效的优势,这些制备的NiFeHCH纳米片可以成为工业用水电解中稀有金属的更好替代品。
更新日期:2019-01-16
中文翻译:

碱性和近中性条件下具有显着OER活性的镍铁羟基碳酸盐水合物的纳米片
评估在OER地区具有与稀有金属相似的活性的铁族金属的独特催化剂对于实现大规模的H 2可持续生产非常重要。在此,我们报道了在反应时间1小时内通过湿化学方法制备的羟基碳酸镍铁水合物(NiFeHCH)的独特纳米片,在碱性和近氧化氢反应中作为氧释放反应(OER)的有效电催化剂-中性媒体。用不同比例的不同浓度的Fe制备NiFeHCH:1:0.2、1:0.4、1:0.6、1:0.8和1:1。其中,发现NiFeHCH(1:0.2)纳米片具有优异的OER活性,需要250 mV的超电势才能达到20 mA cm –2在1 M KOH中具有非常低的Tafel斜率值,即39 mV /十倍频程。具有其他比率的纳米片也具有相当的OER活性,超电势的范围为256至290 mV,Tafel斜率值为39至49 mV /十倍。在1 M NaHCO 3(pH〜8.5)中针对OER筛选的NiFeHCH电催化剂纳米片需要在10 mA cm –2的所有比率范围从389至507 mV和Tafel斜率值从155至205 mV /十年的所有条件下的超电势。 NiFeHCH(1:0.4)纳米片表现出更好的活性,因为在10 mA cm –2时需要389 mV的过电势,而Tafel斜率值为155 mV /十年。凭借这些卓有成效的优势,这些制备的NiFeHCH纳米片可以成为工业用水电解中稀有金属的更好替代品。































 京公网安备 11010802027423号
京公网安备 11010802027423号