当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Divalent Cations on Dark Production of Hydroxyl Radicals from Oxygenation of Reduced Humic Acids at Anoxic–Oxic Interfaces
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-01-16 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00181 Peng Liao , Yuzhen Liang , Zhenqing Shi
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-01-16 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00181 Peng Liao , Yuzhen Liang , Zhenqing Shi
|
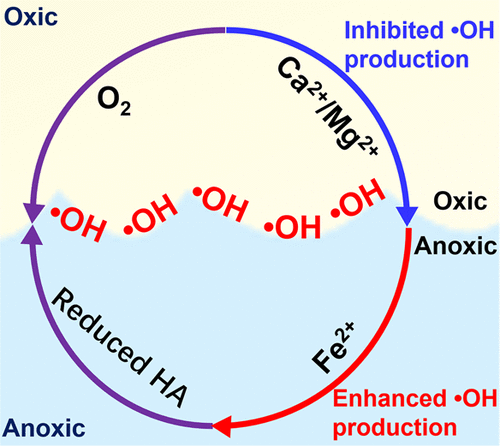
Humic acids (HAs) are redox-active and can serve as either electron acceptors or electron donors to participate in multiple redox reactions. In nature water, HA can be intimately associated with divalent cations, such as Fe2+, Ca2+, and Mg2+, through a series of reactions that may in turn affect the redox reactivity of HA. Recent advances have demonstrated that the oxygenation of reduced HA in the dark can produce ·OH at anoxic–oxic interfaces. However, little is known about the roles of the divalent cations complexed with HA on the production of ·OH from reduced HA. This study provides new knowledge regarding the impact of Fe2+, Ca2+, and Mg2+ ions on the dark production of ·OH from oxygenation of reduced HA at anoxic–oxic interfaces over a wide range of environmentally relevant conditions. Results show that the rates and yields of ·OH production increase with increasing Fe2+ concentration (0.18–0.89 mM). This is largely attributed to the formation of complexed Fe(II) with HA, which increases the number of Fe(II)/Fe(III) cycles and enhances the decomposition of formed H2O2, accelerating the rates of Fenton reactions under circumneutral conditions. However, the promotional effect of Fe2+ on ·OH formation is greatly suppressed in the coexistence of high Ca2+/Mg2+ concentration (5–20 mM), likely due to the retarded formation of HA-Fe(II) complexes and competition of HA surface reactive sites by Ca2+/Mg2+ ions. Findings improve the current understanding of the dark production of ·OH from reduced HA and provide valuable insights toward understanding of carbon cycling and contaminant fate at anoxic–oxic interfaces.
中文翻译:

二价阳离子对还原性腐殖酸在缺氧-氧气界面上的氧化产生的羟基自由基的暗生成的影响
腐殖酸(HAs)具有氧化还原活性,可以作为电子受体或电子供体参与多个氧化还原反应。在自然水中,HA可通过一系列反过来与HA的氧化还原反应性相关的反应与二价阳离子(如Fe 2 +,Ca 2+和Mg 2+)紧密结合。最近的进展表明,在黑暗中,还原的HA的氧合作用可在缺氧-缺氧界面上产生·OH。但是,关于与HA络合的二价阳离子在还原HA产生·OH方面的作用知之甚少。这项研究提供了有关Fe 2 +,Ca 2+和Mg 2+的影响的新知识。在广泛的环境相关条件下,通过在缺氧-缺氧界面上还原的HA的氧化作用而产生的·OH的深色产物中的离子。结果表明,·OH生成速率和产率随Fe 2+浓度(0.18–0.89 mM)的增加而增加。这在很大程度上归因于与HA形成的Fe(II)络合物,增加了Fe(II)/ Fe(III)循环数并增强了形成的H 2 O 2的分解,从而加速了环境温度下Fenton反应的速率条件。但是,高Ca 2+ / Mg 2+共存时,Fe 2+对·OH形成的促进作用被大大抑制。浓度(5–20 mM),可能是由于HA-Fe(II)配合物的形成受阻以及Ca 2+ / Mg 2+离子竞争HA表面反应部位。这些发现改善了目前对还原的HA暗产生·OH的认识,并为了解碳循环和缺氧-含氧界面处的污染物命运提供了宝贵的见解。
更新日期:2019-01-16
中文翻译:

二价阳离子对还原性腐殖酸在缺氧-氧气界面上的氧化产生的羟基自由基的暗生成的影响
腐殖酸(HAs)具有氧化还原活性,可以作为电子受体或电子供体参与多个氧化还原反应。在自然水中,HA可通过一系列反过来与HA的氧化还原反应性相关的反应与二价阳离子(如Fe 2 +,Ca 2+和Mg 2+)紧密结合。最近的进展表明,在黑暗中,还原的HA的氧合作用可在缺氧-缺氧界面上产生·OH。但是,关于与HA络合的二价阳离子在还原HA产生·OH方面的作用知之甚少。这项研究提供了有关Fe 2 +,Ca 2+和Mg 2+的影响的新知识。在广泛的环境相关条件下,通过在缺氧-缺氧界面上还原的HA的氧化作用而产生的·OH的深色产物中的离子。结果表明,·OH生成速率和产率随Fe 2+浓度(0.18–0.89 mM)的增加而增加。这在很大程度上归因于与HA形成的Fe(II)络合物,增加了Fe(II)/ Fe(III)循环数并增强了形成的H 2 O 2的分解,从而加速了环境温度下Fenton反应的速率条件。但是,高Ca 2+ / Mg 2+共存时,Fe 2+对·OH形成的促进作用被大大抑制。浓度(5–20 mM),可能是由于HA-Fe(II)配合物的形成受阻以及Ca 2+ / Mg 2+离子竞争HA表面反应部位。这些发现改善了目前对还原的HA暗产生·OH的认识,并为了解碳循环和缺氧-含氧界面处的污染物命运提供了宝贵的见解。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号