Tetrahedron ( IF 2.1 ) Pub Date : 2019-01-16 , DOI: 10.1016/j.tet.2019.01.020 Kosaku Tanaka , Yusuke Honma , Chihiro Yamaguchi , Lina Aoki , Minami Saito , Momoko Suzuki , Kazuhiro Arahata , Kaoru Kinoshita , Kiyotaka Koyama , Kenichi Kobayashi , Hiroshi Kogen
|
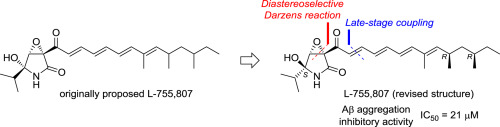
The relative and absolute configurations of L-755,807 were established through total synthesis. All four possible stereoisomers were prepared via a convergent synthetic strategy, including a novel diastereoselective Darzens reaction of an α-alkoxy aldehyde with di-tert-butyl bromomalonate, an E-selective Horner–Wadsworth–Emmons reaction, and late-stage coupling of the ring and side-chain segments. Additionally, biological evaluation of the synthesized compounds revealed their potent inhibitory activities (IC50 = 5–21 μM) against amyloid-β aggregation for the treatment of Alzheimer's disease.
中文翻译:

L-755,807的总合成,立体化学分配和生物学评估
L-755,807的相对和绝对构型是通过全合成建立的。所有四种可能的立体异构体均是通过聚合合成策略制备的,包括α-烷氧基醛与溴化丙二酸二叔丁酯的新型非对映选择性Darzens反应,E-选择性霍纳-沃兹沃思-埃蒙斯反应,以及酯的后期偶联。环和侧链段。此外,对合成化合物的生物学评估表明,它们 对淀粉样β聚集体具有有效的抑制活性(IC 50 = 5–21μM),可用于治疗阿尔茨海默氏病。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号