当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of Prototypic Peptide Transporter DtpA from E. coli in Complex with Valganciclovir Provides Insights into Drug Binding of Human PepT1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-01-15 , DOI: 10.1021/jacs.8b11343
Yonca Ural-Blimke 1 , Ali Flayhan 1 , Jan Strauss 1 , Vasileios Rantos 1 , Kim Bartels 1 , Rolf Nielsen 1 , Els Pardon 2, 3 , Jan Steyaert 2, 3 , Jan Kosinski 1, 4 , Esben M Quistgaard 1, 5, 6 , Christian Löw 1, 5
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-01-15 , DOI: 10.1021/jacs.8b11343
Yonca Ural-Blimke 1 , Ali Flayhan 1 , Jan Strauss 1 , Vasileios Rantos 1 , Kim Bartels 1 , Rolf Nielsen 1 , Els Pardon 2, 3 , Jan Steyaert 2, 3 , Jan Kosinski 1, 4 , Esben M Quistgaard 1, 5, 6 , Christian Löw 1, 5
Affiliation
|
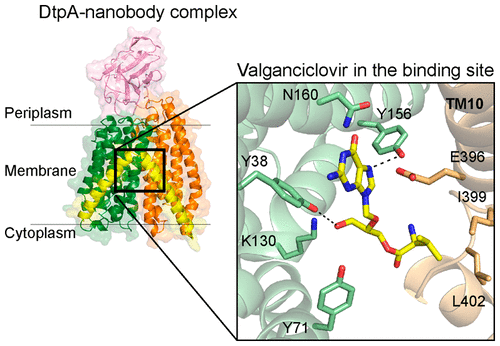
Members of the solute carrier 15 family (SLC15) transport di- and tripeptides as well as peptidomimetic drugs across the cell membrane. Structures of bacterial homologues have provided valuable information on the binding and transport of their natural substrates, but many do not transport medically relevant drugs. In contrast, a homologue from Escherichia coli, DtpA (dipeptide and tripeptide permease), shows a high similarity to human PepT1 (SLC15A1) in terms of ligand selectivity and transports a similar set of drugs. Here, we present the crystal structure of DtpA in ligand-free form (at 3.30 Å resolution) and in complex with the antiviral prodrug valganciclovir (at 2.65 Å resolution) supported by biochemical data. We show that valganciclovir unexpectedly binds with the ganciclovir moiety mimicking the N-terminal residue of a canonical peptide substrate. On the basis of a homology model we argue that this binding mode also applies to the human PepT1 transporter. Our results provide new insights into the binding mode of prodrugs and will assist the rational design of drugs with improved absorption rates.
中文翻译:

来自大肠杆菌的原型肽转运蛋白 DtpA 与缬更昔洛韦复合物的结构提供了对人 PepT1 药物结合的见解
溶质载体 15 家族 (SLC15) 的成员通过细胞膜运输二肽和三肽以及拟肽药物。细菌同源物的结构提供了关于其天然底物的结合和运输的宝贵信息,但许多不运输医学相关药物。相比之下,来自大肠杆菌的同源物 DtpA(二肽和三肽通透酶)在配体选择性方面与人类 PepT1 (SLC15A1) 显示出高度相似性,并转运一组类似的药物。在这里,我们展示了无配体形式的 DtpA 晶体结构(分辨率为 3.30 Å),并与生化数据支持的抗病毒前药缬更昔洛韦(分辨率为 2.65 Å)复合。我们表明缬更昔洛韦出人意料地与更昔洛韦部分结合,模拟经典肽底物的 N 端残基。在同源模型的基础上,我们认为这种结合模式也适用于人类 PepT1 转运蛋白。我们的结果为前药的结合模式提供了新的见解,并将有助于合理设计具有提高吸收率的药物。
更新日期:2019-01-15
中文翻译:

来自大肠杆菌的原型肽转运蛋白 DtpA 与缬更昔洛韦复合物的结构提供了对人 PepT1 药物结合的见解
溶质载体 15 家族 (SLC15) 的成员通过细胞膜运输二肽和三肽以及拟肽药物。细菌同源物的结构提供了关于其天然底物的结合和运输的宝贵信息,但许多不运输医学相关药物。相比之下,来自大肠杆菌的同源物 DtpA(二肽和三肽通透酶)在配体选择性方面与人类 PepT1 (SLC15A1) 显示出高度相似性,并转运一组类似的药物。在这里,我们展示了无配体形式的 DtpA 晶体结构(分辨率为 3.30 Å),并与生化数据支持的抗病毒前药缬更昔洛韦(分辨率为 2.65 Å)复合。我们表明缬更昔洛韦出人意料地与更昔洛韦部分结合,模拟经典肽底物的 N 端残基。在同源模型的基础上,我们认为这种结合模式也适用于人类 PepT1 转运蛋白。我们的结果为前药的结合模式提供了新的见解,并将有助于合理设计具有提高吸收率的药物。







































 京公网安备 11010802027423号
京公网安备 11010802027423号